Volume 5, Issue 8
August 2025
Neurogenic Inflammation and Its Role in Periodontal Disease
Hanan Abdullah AlMozher, Najla Abdurahman Almuayli, Majed Mohammad Almohaimeed
DOI: http://dx.doi.org/10.52533/JOHS.2025.50805
Keywords: Periodontal Disease, Periodontitis, Neurogenic Inflammation, Neuroinflammation, Neuropeptides, Neurodegenerative Diseases
Periodontal disease is an inflammatory disease caused predominantly by bacteria. Its prevalence is high among adults, especially mild and moderate forms. Periodontal disease is considered the second most widespread oral disease after dental caries. Periodontal prevalence can be affected by age, gender, ethnicity, and socioeconomic status. Furthermore, factors such as smoking, obesity, diabetes mellitus, and metabolic syndrome contribute strongly to periodontal disease. Neurogenic inflammation is involved in a variety of systemic diseases, including periodontitis. However, the role of neuroinflammation in periodontal diseases is unclear. Thus, this review aims to discuss the mechanistic pathways and therapeutic implications of neurogenic inflammation in periodontal diseases. Multiple studies found an association between neuropeptides and the development of periodontal diseases, as they found increased levels of neuropeptides in tissues and plasma of diseased patients. Studies also found a link between periodontal diseases and neurodegenerative diseases, including multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease. Understanding these interactions may provide new therapeutic targets and improve both oral and systemic health outcomes.
Introduction
Periodontal disease is an inflammatory condition, primarily caused by bacterial plaque, and leads to the destruction of periodontal tissues. It is a leading cause of tooth loss and has been associated with multiple systemic conditions in both developing and developed countries. Periodontal disease is highly prevalent in adults globally, particularly its mild and moderate forms, with prevalence rates around 50% (1). The incidence of severe forms of this disease increases especially between the third and fourth decades of life, with a prevalence around 10% globally (2). The prevalence of periodontal disease rises with age, and in the United States, 70.1% of adults aged 65 and older are affected by some form of the condition, making it the second most widespread oral disease after dental caries (3).
Ethnicity, gender, and socioeconomic status can also affect the prevalence of periodontitis. Furthermore, factors such as smoking, obesity, diabetes mellitus, and metabolic syndrome contribute strongly to periodontal disease (4). The major periodontitis pathogens include Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Treponema denticola, Tannerella forsythia, Prevotella intermedia, and Fusobacterium nucleatum (5).
Although bacteria are an established cause of periodontitis, they alone do not fully lead to the advanced destruction of periodontal tissues. An important yet often overlooked aspect of periodontitis is its neurogenic component (6). The involvement of neurogenic inflammation in a variety of systemic diseases is well documented, and its role in periodontitis has also been suggested. The concept of neurogenic inflammation was first introduced by Jancsó and Szolcsányi in 1967 (7).
Although neurogenic inflammation is considered a protective mechanism, excessive or chronic activation may result in tissue damage instead of repair. When chemical irritants interact with receptors on sensory nerves, they can trigger the release of neuropeptides such as Substance P (SP), leading to neurogenic inflammation (8). Neurons produce these bioactive molecules, known as neuropeptides or peptide neurotransmitters, which exert their biological effects through extracellular receptors on target cells (9). However, the role of neurogenic inflammation in periodontal diseases and its contribution to neurodegenerative diseases is unclear. This review aims to explore current evidence focused on neurogenic inflammation in periodontal diseases, highlighting its contribution to neurodegenerative diseases.
Methods
A comprehensive literature search was conducted in Medline (via PubMed), Scopus, and Web of Science databases up to June 16, 2025. Medical Subject Headings (MeSH) and relevant free-text keywords were used to identify synonyms. Boolean operators (AND, OR) were applied to combine search terms in alignment with guidance from the Cochrane Handbook for Systematic Reviews of Interventions. Key search terms included: “Periodontal Disease” OR “Periodontitis” AND “Neurogenic Inflammation” OR “Neuroinflammation” OR “Neuropeptides”. Summaries and duplicates of the found studies were exported and removed by EndNote X8. Any study that discusses the role of neurogenic inflammation in periodontal diseases and is published in peer-reviewed journals was included. All languages are included. Full-text articles, case series, and abstracts with related topics are included. Case reports, comments, and letters were excluded.
Discussion
Role of Neurogenic Inflammation in Periodontal Disease
The pathogenesis and causative mechanisms of periodontal diseases are multifactorial, with neurologic inflammation playing a key role in these mechanisms. Multiple studies found an association between neuropeptides and the development of periodontal diseases, as they found increased levels of neuropeptides in tissues and plasma of diseased patients.
Neuroinflammation is regulated by the innate immune system and includes two forms: acute and chronic (3). The acute form is characterized by a transient increase of inflammatory markers, while the chronic form involves persistent, low-grade inflammation and delayed resolution. Chronic neuroinflammation leads to prolonged cytokine release, especially from glial cells, which leads to neuronal damage and cell death (3). Unresolved systemic inflammatory stimulation can lead to chronic microglial activation, resulting in microglial hypersensitivity to subsequent stimuli (10).
Toll-like receptor 4 is a part of a key pathway that, when activated by lipopolysaccharide (LPS), stimulates proinflammatory signaling in astrocytes and microglia (11). These cascades are significant in periodontal diseases, since they lead to an increase in systemic inflammatory markers, such as C-reactive protein (CRP) (12). Thus, sustained systemic inflammation in periodontal disease may play a role in initiating or exacerbating neuroinflammatory processes.
A previous study found an association between elevated levels of specific periodontal pathogens and increased serum proinflammatory cytokines. For example, the study reported an association between Aggregatibacter actinomycetemcomitans and high serum IFN-γ and between Porphyromonas gingivalis and high TNF-α levels (13). Therefore, different bacterial species can stimulate systemic immune systems by activating different lymphocyte populations, given that each immune cell type produces distinct cytokines (3).
Furthermore, the central nervous system (CNS) can be significantly affected by prolonged exposure to inflammatory agents, since they can disrupt the blood-brain barrier (BBB). This disruption of the BBB allows microbial components and cytokines to reach the brain via the bloodstream or cranial nerves (3). For instance, proinflammatory cytokines from peripheral inflammation may stimulate the vagus nerve that transmits the inflammatory signal to the brain (14). Leptomeningeal cells, located within the meninges, further contribute to signal transduction from peripheral immune cells like macrophages to the brain’s microglia (15). Notably, microbial invasion and neurogenic inflammation can lead to neurodegenerative disorders, as multiple postmortem analyses have detected DNA from periodontal pathogens such as P. gingivalis and Treponema denticola in the brains of Alzheimer’s disease (AD) patients (16, 17).
Multiple previous animal studies supported these findings. A study injected LPS peripherally into mice, resulting in systemic inflammation and inducing the expression of TNF-α and IL-1β in the brain. More intense neuroinflammatory responses and behavioral impairments were observed in older animals, mirroring cognitive declines observed in the elderly experiencing systemic infections (18). Another study induced periodontitis in mice using ligature placement and reported increased proinflammatory cytokines both in gingival tissue and the brain (19), suggesting that oral inflammation can induce a neuroinflammatory response. Furthermore, even in the absence of live pathogens, ligature-induced periodontitis in wild-type mice altered microglial activation and brain cytokine profiles. In 5×FAD mice, a transgenic Alzheimer’s model, this model led to a significant reduction in plaque-associated microglia, further implying that periodontitis-induced inflammation affects AD pathology (20).
Gut microbiome disruption is another emerging mechanism linking periodontitis and neurogenic inflammation (3). Oral microbes were observed in fecal samples, suggesting an oral-gut translocation. This translocation can impair the gut-brain axis, which is a complex system through which the gut microbiota regulates neurological function via metabolic, immune, and hormonal pathways. An increase in BBB permeability may occur due to the disruption of gut hemostasis, which allows the entry of microbial metabolites or inflammatory signals to the CNS (21).
A study suggested a theory that sporadic Parkinson’s disease (PD) may arise in the nasal cavity or gut, with pathology progressing along the vagal or olfactory nerves. This theory follows the “leaky gut–leaky brain” hypothesis, which is that the increase in intestinal permeability allows the microbial components to reach the brain and results in neurodegeneration. These effects may result from the modulation of systemic immune and biochemical pathways or through direct migration across the cerebrospinal fluid or BBB barriers (21).
Therefore, periodontal disease is associated with chronic microbial dysbiosis, which leads to cognitive impairment via the TLR4/MyD88/NF-κB signaling pathway. This supports the model of an oral–gut–brain axis (22). A study by Chi et al. supported these findings as they showed that oral administration of P. gingivalis in mice resulted in both gut dysbiosis and cognitive impairment (23). Furthermore, the study demonstrated that P. gingivalis disrupted the glymphatic system, which is a crucial waste-clearance pathway in the brain, thus decreasing its ability to remove metabolic byproducts such as amyloid-beta, a key hallmark of AD. Another systematic review evaluated the impact of neurologic inflammation on periodontal diseases (6). In patients with gingivitis and periodontal-affected sites, tissue samples showed the presence of neurochemical markers, suggesting the contribution of neuropeptides in the etiopathogenesis of periodontal diseases (24).
Inflammatory Neuropeptides in Periodontal Disease
Multiple studies explored the link between neuropeptides and periodontal diseases; an overview of these neuropeptides is shown in Table 1. These studies found that SP was higher in the periodontitis sites than in healthy sites (25-27). Furthermore, studies reported that SP was positively associated with neurokinin A (NKA) clinical measurement, indicating their impact on periodontal disease severity. SP, among other pro-inflammatory neuropeptides, can induce lymphocyte infiltration (28) and stimulate interleukin-2 production (29). SP and NKA are resistant to degradation by carboxypeptidase, which allows their levels to remain elevated in periodontitis (25). Notably, a study reported a significant increase in NKA-like immunoreactivity and SP-like immunoreactivity in periodontitis and gingivitis sites compared to healthy sites (27).
Various factors cause the elevation of SP levels in periodontal diseases. One contributing mechanism is SP's ability to enhance osteoclast activity, thereby promoting bone resorption (30). The detection of SP-like immunoreactivity before neutrophil infiltration indicates its role in the early phases of inflammation (31). SP may facilitate leukocyte infiltration through various pathways, a critical component of the inflammatory process (26). Clinical studies have also reported a significant decrease in pro-inflammatory neuropeptides like SP following periodontal treatment (26).
Calcitonin gene-related peptide (CGRP) is another outcome parameter measured to assess the influence of neurologic inflammation on periodontal disease. Studies showed that CGRP levels are higher in healthy tissues than in periodontal-affected tissues (25). This can be attributed to the CGRP’s inhibitory effect on lymphocytic proliferation (32) and IL-2 production (33), resulting in inhibition of osteoclastic bone resorption and stimulation of osteogenesis. This result can also be explained by the rapid breakdown of CGRP due to carboxypeptidase activity in the gingival crevicular fluid obtained from periodontitis sites. This is important because this neuropeptide has the ability to suppress osteoclastic bone resorption and promote osteogenesis (25). However, two previous studies noticed no clear differences between SP and CGRP levels in periodontitis-affected sites and clinically healthy sites (24, 34).
Vasoactive intestinal polypeptide (VIP) is a macrophage deactivating factor that impedes the excessive production of pro-inflammatory factors (35) and inhibits the production of LPS-induced tumor necrosis factor-alpha, IL-12, and IL-6 in activated macrophages (36). Studies reported that VIP levels were reduced after the induction of periodontal treatment. However, VIP levels may increase due to its anti-inflammatory role (as seen in clinically healthy sites).
This increase in VIP periodontitis sites may be caused by the LPS stimulation effect on the production and secretion of VIP in vitro (37). Thus, it appears that SP and VIP have antagonistic effects on periodontal inflammation. Maintaining a balance between pro- and anti-inflammatory neuropeptides is a crucial aspect of the host immune response.
|
Table 1. Overview of Neuropeptides Involved in Periodontal Disease |
|||
|
Neuropeptide |
Inflammatory role |
Functions |
Location |
|
Substance P (SP) |
Pro-inflammatory |
Vasodilator |
Peripheral nerves, including enteric neurons and capsaicin-sensitive primary afferent neurons. |
|
Calcitonin gene-related peptide (CGRP) |
Anti-inflammatory |
Vasodilator |
Distributed throughout the central and peripheral nervous systems. |
|
Neurokinin-A (NKA) |
Pro-inflammatory |
Increases vasodilatation, microvascular permeability, and plasma extravasation. |
Peripheral nerves, including enteric neurons and capsaicin-sensitive primary afferent neurons. |
|
Neuropeptide Y (NPY) |
Pro-inflammatory |
Vasoconstrictor |
Distributed throughout the central and peripheral nervous systems. |
|
Vasoactive intestinal polypeptide (VIP) |
Anti-inflammatory |
An immunomodulatory peptide that regulates the production of pro- and anti-inflammatory mediators, relaxes smooth muscle, and induces salivary secretion. |
In the central and peripheral nervous systems. |
Periodontitis and Neurodegenerative Diseases
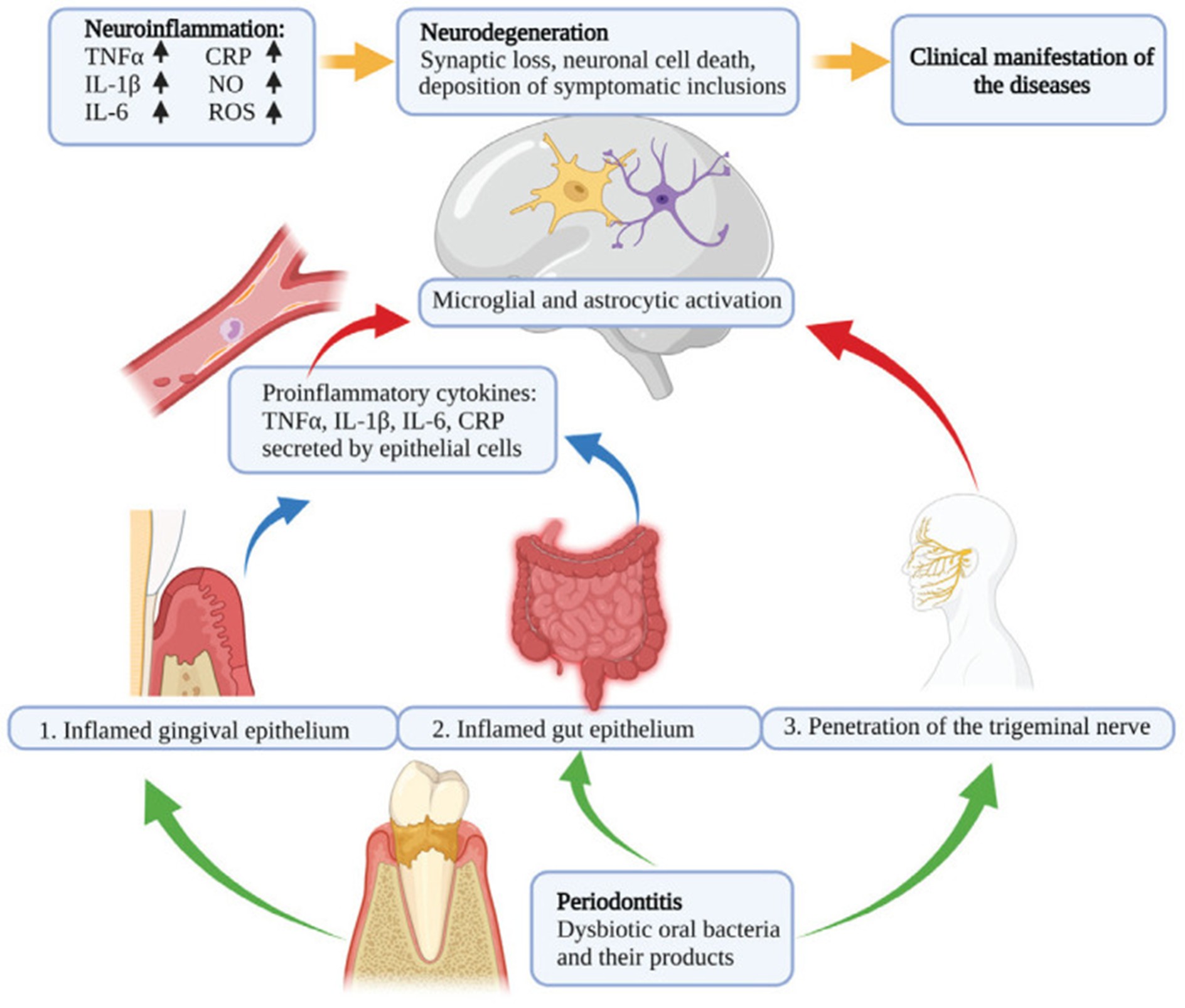
Recently, multiple studies have demonstrated a link between periodontal diseases and neurodegenerative diseases, including multiple sclerosis (MS), Parkinson’s disease, and Alzheimer’s disease. This link is regulated through mechanisms involving systemic inflammation and microbial translocation. The pathophysiological link between periodontal disease, neuroinflammation, and neurodegenerative diseases is shown in Figure 1.
Multiple sclerosis is an autoimmune demyelinating disease with various environmental and genetic triggers, which may be worsened by systemic inflammation. Moreno et al. reported that peripheral inflammation induced by LPS may worsen axonal damage in experimental autoimmune encephalomyelitis rats (38). Another study demonstrated that oral P. gingivalis exacerbated MS symptoms in mice induced by myelin oligodendrocyte glycoprotein, highlighting a link to periodontal infection (39). However, epidemiological studies exploring the link between periodontal diseases and MS showed mixed results. A study in Taiwan demonstrated a female-specific association between periodontitis and MS (40), while a study in Norway observed no significant link (41).
Alzheimer’s disease is associated with neurofibrillary tangles and β-amyloid plaques, resulting in neuronal and synaptic loss in important brain areas, including the hippocampus and entorhinal cortex. It is manifested by disorientation, memory deficits, language processing decline, and impaired judgment. Elevated systemic inflammatory markers such as IL-6, IL-1β, TNF-α, and CRP have been associated with increased dementia risk (42). Additionally, complement proteins were detected in amyloid plaques (43), supporting the role of immune mechanisms in AD.
Periodontal diseases may lead to the entry of proinflammatory mediators and microbial agents into the brain through the trigeminal nerve or bloodstream, contributing to the development of AD. P. gingivalis and T. denticola have been found in postmortem AD brain tissues (16, 17). P. gingivalis proteases were also linked to tau pathology (17). In AD mouse models, oral P. gingivalis exposure impaired cognition and increased amyloid deposition and proinflammatory cytokines (20). In wild-type mice, gingipain exposure led to neuronal degeneration and AD-like pathology (44). Other studies further confirmed cognitive impairments and memory deficits in periodontitis-induced rodent models (45).
Parkinson’s disease is characterized by degeneration of dopaminergic neurons in the substantia nigra and formation of Lewy bodies composed of α-synuclein. PD can cause motor dysfunction and cognitive deficits. In PD brains, neuroinflammation is characterized by microglial activation and elevated cytokines such as IL-1β, IL-6, and TNF-α (46). Notably, patients with PD usually struggle with oral hygiene, increasing their susceptibility to periodontitis (47).
Recently, multiple studies found an association between periodontal diseases and PD. In their retrospective cohort study, Chen et al. reported a higher risk of PD in patients with periodontitis (48). Follow-up research was conducted and showed that dental scaling reduced PD risk (49). Furthermore, new-onset PD was associated with tooth loss (50). Another study identified P. gingivalis gingipain R1 in blood clots from PD patients, highlighting its potential role in systemic inflammation and hypercoagulation (51). In a PD mouse model with the LRRK2 R1441G mutation, oral P. gingivalis increased α-synuclein, reduced dopaminergic neurons, and elevated microglial activity, supporting a causal link (52).
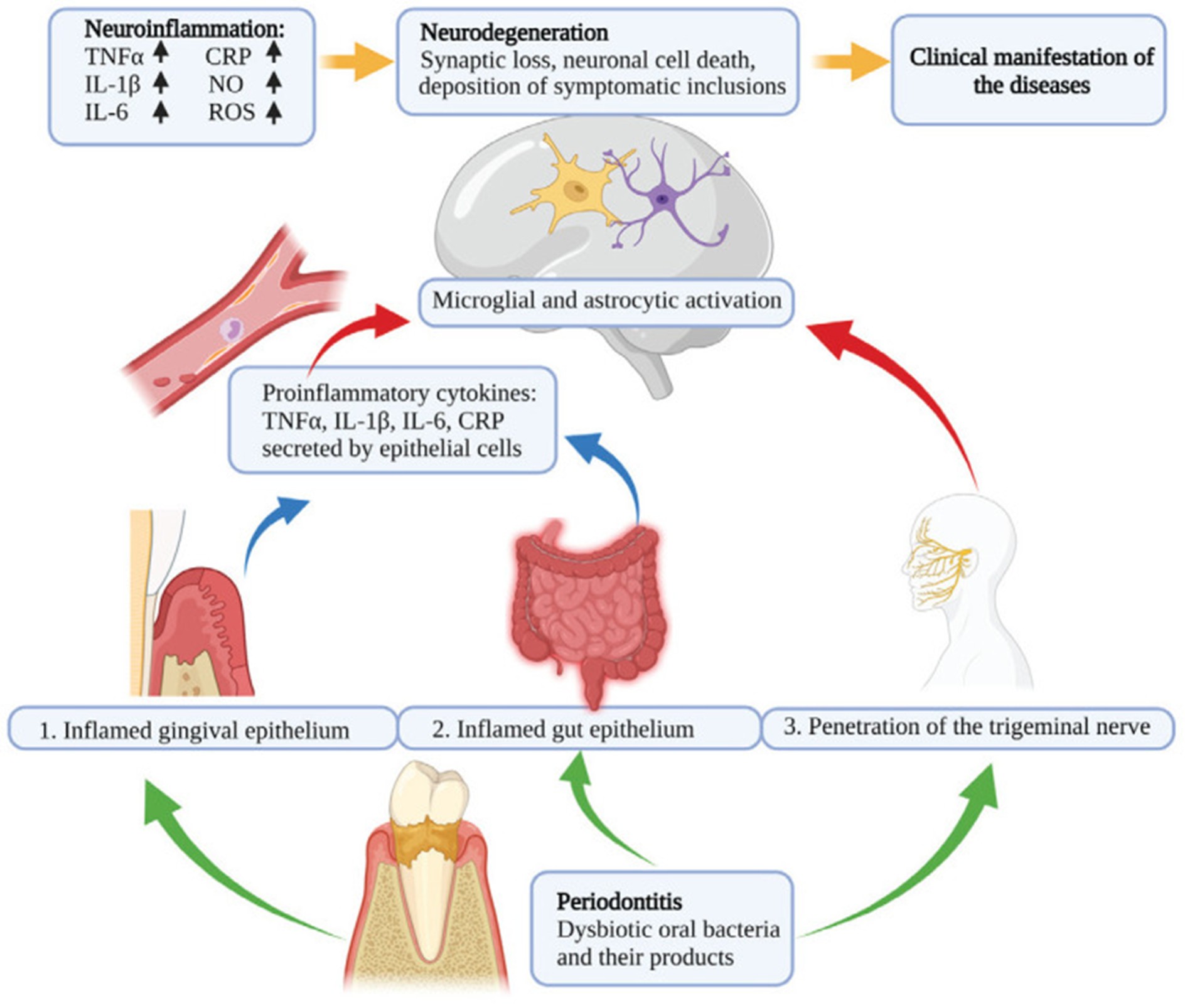
Figure 1: Pathophysiological link between periodontal disease, neuroinflammation, and neurodegenerative diseases (3).
Periodontal Disease Therapy
Periodontal therapy aims to treat infection and inflammation to suppress tissue destruction. However, even after stabilization of the disease, tissue regeneration remains limited depending on age, defect type, and systemic health (53). Periodontal therapy may include surgery or adjunctive antimicrobials in severe cases, especially when mechanical debridement alone is inadequate (5). Notably, smoking can worsen outcomes; thus, its cessation is critical to treatment success (54), likely due to shifts in the subgingival microbiota.
Although treatment reduces pathogen levels, recolonization may occur over time. Therefore, oral hygiene and professional maintenance are crucial to enhance treatment outcomes (55). In severe cases with tooth loss and bite dysfunction, complex prosthodontic rehabilitation is usually required; nevertheless, patients with periodontitis who underwent reconstructions remain at higher risk for tooth loss, influenced by factors such as socioeconomic status, age, diabetes, and non-compliance (56).
Periodontal therapy can also improve systemic low-grade inflammation. Furthermore, while intensive therapy may initially and transiently affect endothelial function negatively, long-term improvement occurs after 6 months (57). Treatment also lowers inflammatory biomarkers (e.g., IL-6, TNF-α) in patients with cardiovascular disease and diabetes (58) and improves glycemic control in diabetics (59). Periodontal treatment should be personalized and based on disease mechanisms and host response. Future strategies also should involve inflammation modulators and immunotherapies, tailored using omics technologies to optimize individual outcomes (60).
Conclusion
Emerging evidence underscores the significant role of neurogenic inflammation in the pathogenesis of periodontal disease. Neuropeptides such as Substance P, CGRP, and VIP appear to mediate key inflammatory responses that influence periodontal tissue destruction and systemic inflammation. Moreover, growing data support a link between periodontitis and neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and multiple sclerosis, through mechanisms involving microbial translocation and immune activation. Understanding these interactions may provide new therapeutic targets and improve both oral and systemic health outcomes.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding.
Ethical considerations
This study is a systematic review of previously published literature and does not involve any original data collection involving human or animal subjects. Therefore, ethical approval was not required.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection, analysis and final writing of the manuscript.