Volume 5, Issue 4
April 2025
Ultrastructure Study of Anterior Cruciate Ligament of Advanced Osteoarthritis Patient Subjected to Total Knee Replacement Surgery
Adel Hegaze
DOI: http://dx.doi.org/10.52533/JOHS.2025.50401
Keywords: ACL, collagen fibril, ultrastructure, knee OA, microscopic examination
Background: The Anterior Cruciate ligament (ACL) has the highest percentage of elastic fibers. It is one of the most common affected ligaments in sport injuries. Osteoarthritic patients often present to the clinic with painful ACL.
Case Reports: ACLs were collected from two patient and a total of four knees. The composition of ligament as regard the collagen fibrils, collagen organization, density of fibroblasts and blood vessels, and distribution of the collagen type I, III and V fibrils were examined.
Results: The ultrastructural cellular features of the tibial stump of the ruptured ACL showed the presence of myofibroblasts.
Conclusion: The finding has a potential bearing on the healing capacity of the ruptured stump and on the functional outcome of the remnant-preserving ACL reconstruction. In addition, the fibril diameter range and collagen fibril index were similar to the previous studies despite of the age effect.
Introduction
The tendons are strong, relatively inextensible structures They are composed of closely packed collagen bundles in a matrix of proteoglycan (1). Fibroblasts are the predominant cell type and are arranged in parallel rows between bundles of collagen fibers (2, 3). In general, Anterior cruciate ligament (ACL) shows the highest percentage of the elastic fibrils, fibroblasts and blood vessels and a relatively low fibrils/ interstitial ratio. Anterior cruciate ligament (ACL) tears are very common injuries, especially in sports (4). Even though the incidence of developing osteoarthritis increases after ACL injury and reconstruction, (5, 6) it is important to determine if osteoarthritis itself can have changes on ACL.
Osteoarthritis (OA) is the most common form of arthritis globally. OA have many predisposing risk factors such as age, gender, joint injury, obesity, and muscle weakness. It occurs by exerting a mechanical stress by abnormal joint movement leading to increase pro-inflammatory markers in the joint which mediates the joint destruction (7). A number of microscopic changes start taking place in the joint starting with collagen matrix damage which leads the chondrocytes to proliferate and form clusters. Progression of the disease also causes soft-tissue damage affecting ligaments, menisci and the joint capsule (8). It has been estimated that around 23% of painful osteoarthritis have ACL deficiencies with almost half of them not reporting any previous ACL injury (9). The normal anterior cruciate ligament (ACL), which provides primary restraint of the anterior movement of the tibia, its structure consisting of collagen bundles that respond to a wide range of motion and torsional movements of the knee joint. A major part of the ACL is composed of bundles of type I collagen. Histologically, the structure of the ACL consists of collagen and elastin embedded in a proteoglycan–water matrix. Collagen is the main component of the tendon with collagen fibrils accounting for 65% to 75% of its dry weight (10). The ACL provides a crucial part in the mechanical integrity of the knee movement; it is commonly sacrificed in Total knee replacement (TKR) procedures. It is also known that ACL integrity is affected by OA most likely due to intercondylar notch osteophytes (11). In this study, we examined the histological features of the ACL inpatient subjected to total knee replacement due to advanced OA changes.
Case Approach
Study settings
Two patients were selected with grade IV OA. One male and one female aged 72 and 75, respectively. Both patients had diabetes and hypertension and were non-smokers with no history of trauma. Specimens were obtained at the operation theatre when they underwent bilateral TKR from the knees of the two patients. Both patients had OA for a duration of 3 years and were treated medically with no previous surgical interventions and no history of previous sport injury or trauma.
Histology
All tissues were investigated by light and electron microscopy, immunohistochemistry and were additionally analyzed by morphometry. For light microscopy the removed specimens were formalin fixed [4% formalin in phosphate-buffered saline (PBS)], paraffin-embedded via routine procedure. The size of all the probes studied were 20 mm, 10 mm whole width and 9 whole thickness of tendons for the patellar tendon and quadriceps and 20 mm 10 whole width and 9 whole thickness for the tendons and the ACL. Transmission electron microscopy tendons were placed in 4% paraformaldehyde for 24 hours then were transferred to 4% formalin. Afterwards the samples were cut and embedded for transmission electron microscopy. The cross sections (100 nm thick) of each fixed tendons were rinsed in 0.1 M phosphate buffer then placed in 1% osmium tetroxide in 0.1 M phosphate buffer for 2 hours. They were then dehydrated in graded ethanol solutions (30, 50, 65, 75, 95 and 100%) and transferred to propylene oxide. The tendons were infiltrated with an Epon and the infiltration process was performed for 24 hours and the specimens were hardened at 40? C for 48-hours. Thin sections, three from the center of each tendon studied, were stained with aqueous uranyl acetate and lead citrate, and then scanned with Zeiss TEM. Ten random fields were photographed from each section at a magnification of 10,0000. Four groups of tissue samples (patellar, quadriceps, semitendinosus and gracilis) and the ACL were collected, and processed for measurement of collagen fibril diameter, fibril density (fibril-interstitium ratio), density of blood vessels, density of fibroblasts, percentage of elastic fibrils and percentage of collagen type I, III and V.
The microstructures of the anterior cruciate ligament (ACL) were studied. The purpose of this study was to clarify the microstructural contracture of the ACL with the advanced OA knee. The ACLs of four fresh specimens were harvested from patients subjected to total knee replacement. The ACL usually excised and discarded during the steps of surgical procedure. The ACL bundle examined for ultrastructure’s contracture. It showed thick, unidirectionally oriented fibrils.
Method of scanning electron microscope examination of tissue
Two 0.5 to 1 cm samples were-taken from the tissue and fixed in 5 cold buffered glutaraldehyde for two days. The samples were then washed by cacodylate buffer three times, 30 minutes for each, and post fixed in 1 % osmium tetroxide for two hours. Samples were then washed in cacodylate buffer for three times, 30 minutes each, and then dehydrated by using ascending series of ethanol 30,50,70,90 for two hours, 100% for two days, and then to amyl acetate for two days. Critical point, drying was applied to the samples by using liquid carbon dioxide. Each sample was fixed on a metallic block by using silver paint. By using gold sputter coating apparatus, samples were evenly gold coated in a thickness of 15nm. Samples were examined by uSIng JEOL JSM 5400 LV scanning electron microscope 15- 25.kv and photographed.
Method of T.E.M processing
Total of 5 to 10 small pieces of 1x2 mm in size were taken from each specimen and fixed in 5 % cold glutaraldehyde immediately after dissecting the animal for 24–48 hours. The specimens were then washed in cacodylate buffer (pH 7.2) 3 to 4 times for 20 minutes each time and post fixed in 1 % O4 s4 for 2 hours. Afterwards, it was washed in the same buffer four times. Dehydration by ascending grades of alcohol 30 – 50 – 70 – 90 and 100% 2 hours of each were done and were embedded in epon – araldite mixture according to the protocol of E.M. unit Assiut Uni (12). From the embedded blocks semithin sections by L K B ultramicrotome in thickness of 0.5 – 1 micron were prepared for orientation of the tissue and photographed by sc30 Olympus camera and then ultrathin section in thickness of 500 – 700 A were made using leica AG ultramicrotome and contrasted in uranyl acetate and lead citrate. It was examined by JEM 100 CXII electron microscope at 80 KV and photographed by CCD digital camera Model XR- 41.
I-2- ACL (Ligament):
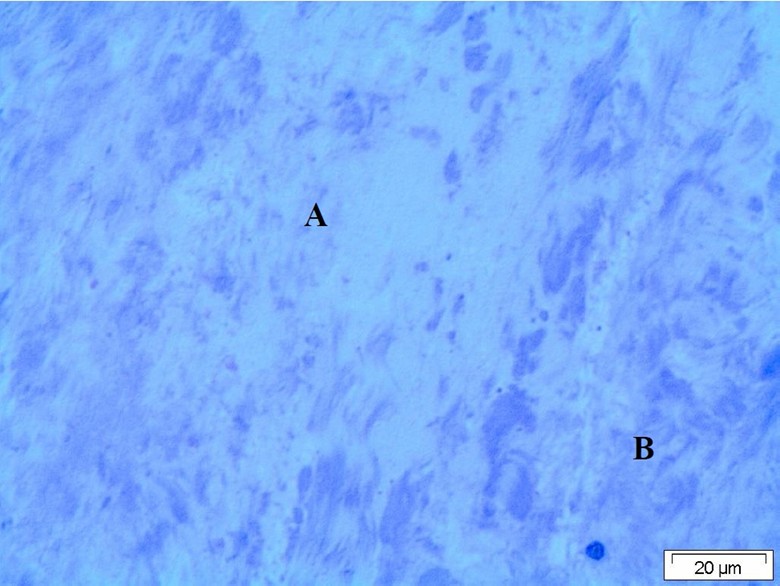
Light microscopy of the ligament revealed presence of moderate degenerative changes of the fibrous tissue forming the ligament. The tissue of the ligament appeared partially of normal structure inform of bundle of collagen fiber and the other parts were degenerated and appeared faintly stained with fragmentation of the collagen fiber (Figure 1).
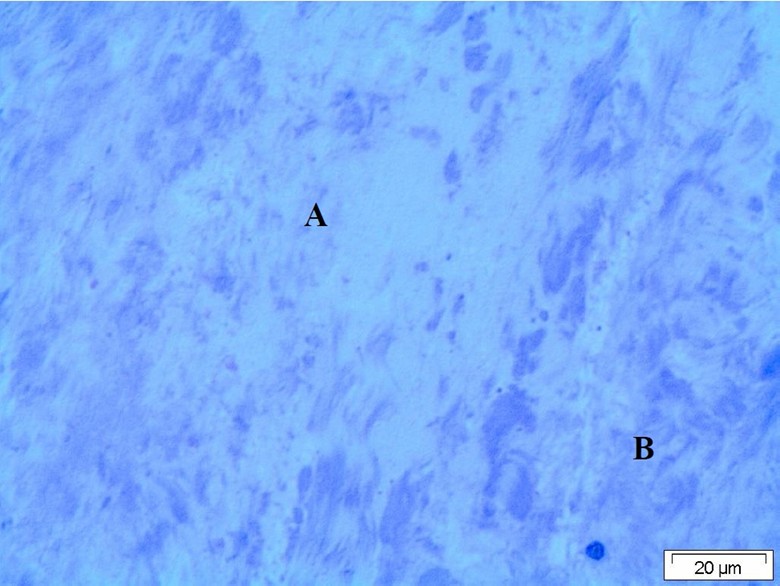
Figure 1: Light micrograph of the ligaments showing degenerated collagen fiber. A: Normal structural area, B: Ziehl–Neelsen staining.
Transmission electron microscopy of the ligament showed presence of area of normal structure of connective tissue which formed by collagen fiber (Figure 2). In other area, the collagenous connective tissue appeared edematous contain beside the collagen fiber, fibroblast, macrophages, and mast cells having in their cytoplasm large amount of electron dens granules and dilated vesicles (Figure 2). In other areas, the collagen fiber degenerated and fragmented with deposition of light electron dens material in between (Figure 2). In the affected areas, the presence of fibroblasts, which appeared large having nucleus and their cytoplasm, contain numerous fat globules and small electron dens lysosomes. These cells represent surface processes and are imbedded or surrounded by light electron dens matrix (Figure 2). Also, some of these cells lost their structure, fragmented, and calcified and embedded in the light electron dens matrix (Figure 2).
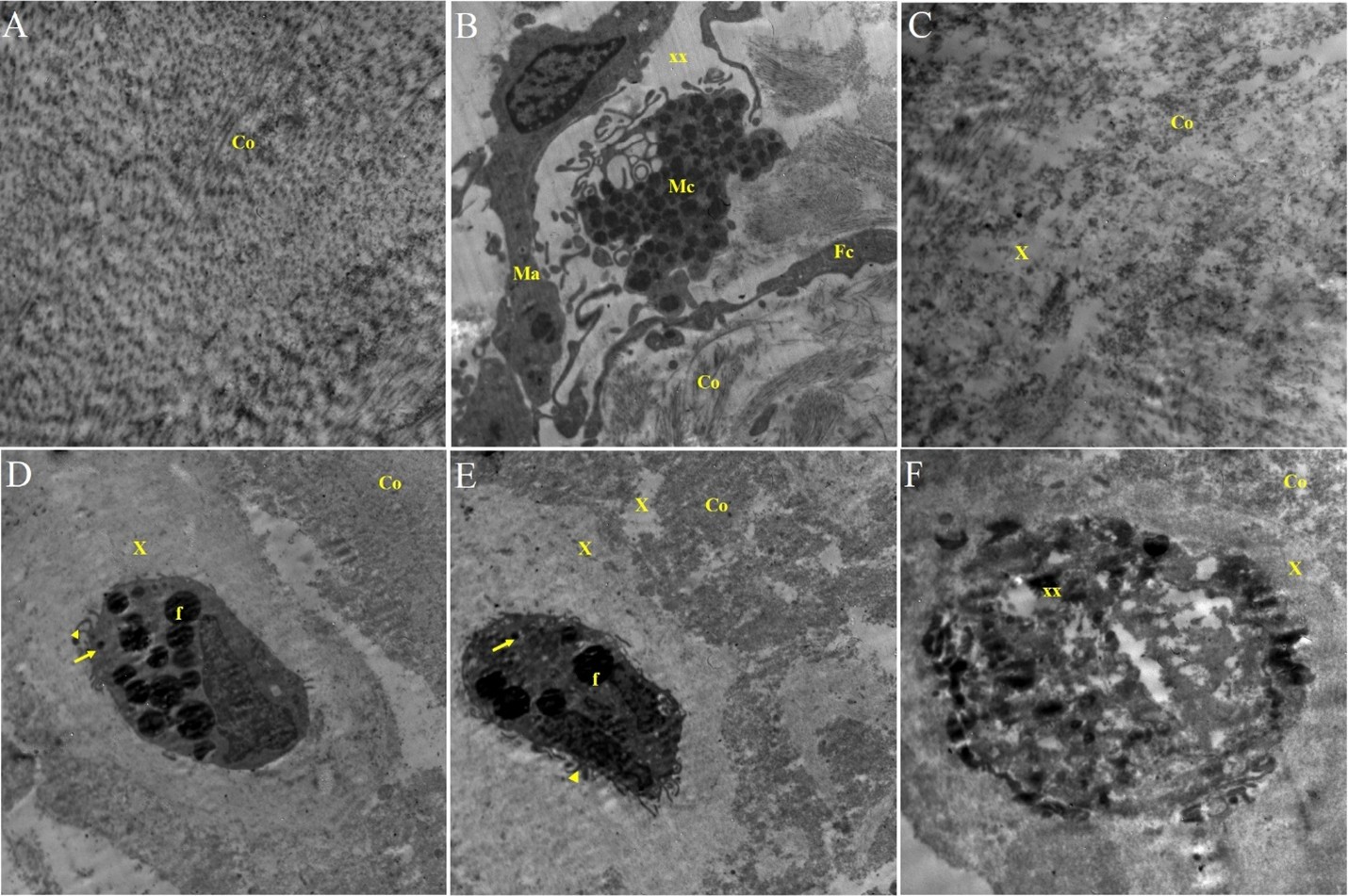
Figure 2: A) Transmission electron (T.E) microscopy showing the normal ultra-structure of the collagen connective tissue fiber of the ligaments (Co). B) T.E. micrograph of the ligaments showing presence of fibroblasts (Fc), collagen fibers (Co), edema (xx), mast cell (Mc) and macrophage (Ma). C) T.E. micrograph of the ligament showing fragmentation (xx) of the collagen fibers (Co) with deposition of light electron dens material in between (X). D&E) T.E. micrograph of the ligaments showing fibroblasts contain fat globule (f), lysosomes (arrow) short surface processes (arrowhead) embedded in light electron dens matrix (X), collagen fiber (Co). F) T.E. micrograph of the ligaments showing fragmented fibroblasts with calcification surrounded by light electron dens matrix (X), degenerated collagen fiber (Co).
II-2-ACL (Ligament):
Light microscopy of the ligament revealed that the ligaments formed by collagenous connective tissue. The collagen fibers degenerated and the fibrocystic cells deeply stained and surrounded by hallow zoon (Figure 3).
Transmission electron microscopy of the ligament revealed presence of fibroblasts having electron dens nucleus and their cytoplasm contain numerous dilated vesicles contain light electron dens material and representing surface processes variable in their length. These cells embedded in matrix similar in electron density to that found in the dilated vesicles (Figure 4). The collagen fiber related to these cells were degenerated and contain electron dens calcified granules (Figure 4). Some of these fibroblast cells contain lipofuscin granules or pigment (Figure 4). Also, in other areas the collagenous connective tissue of the ligaments beside degeneration and fragmentation of the collagen fibers, macrophages, lymphoid and mast cell infiltration, and edema were observed (Figure 4).
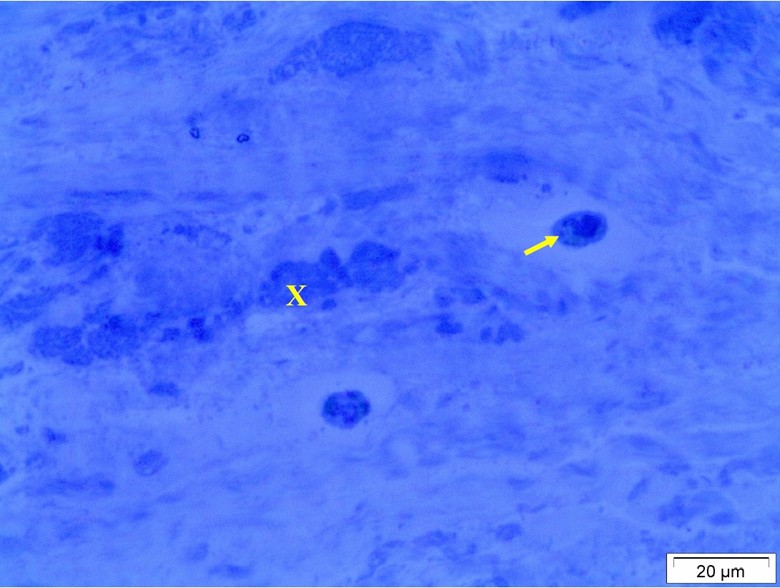
Figure 3: Light micrograph of the ligaments showing degeneration of the collagen fibers (X) and presence of deeply stained cells surrounded by hallow zoon (arrow).
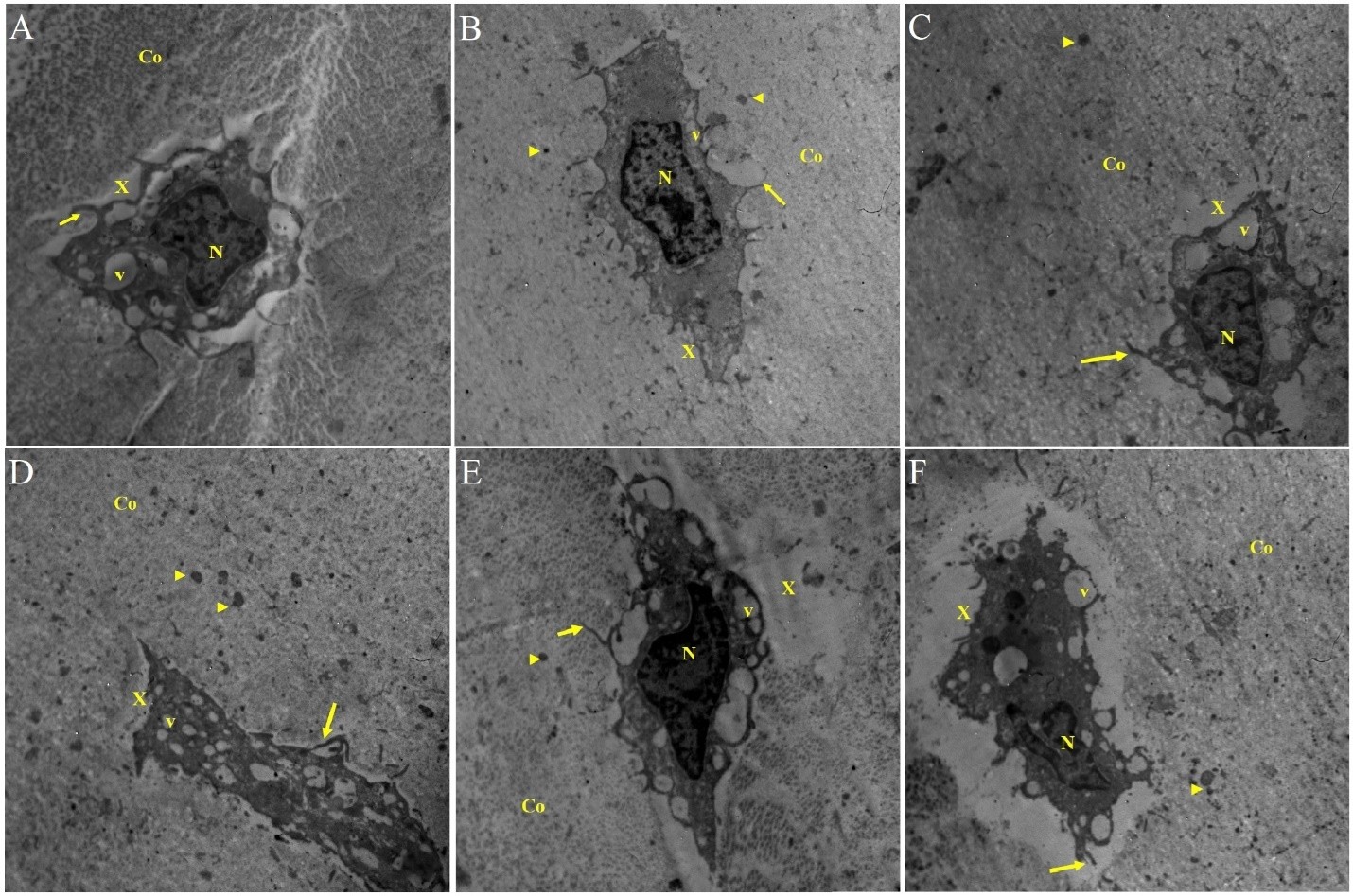
Figure 4: A) Light micrograph of the ligaments showing degeneration of the collagen fibers (X) and presence of deeply stained cells surrounded by hallow zoon (arrow). B-F) T.E. micrograph of the ligament showing presence of fibroblasts having electron dens nucleus (N), numerous dilated vesicles contain light electron dens material (v) representing surface processes (arrow), embedded in matrix (X), presence of calcified granule (head arrow) in degenerated collagen (Co). A-F) T.E. micrograph of fibroblasts of the ligament contains electron dens nucleus (N), dilated vesicles (v), lipofuscin granules (g), surface processes (arrow) and embedded in matrix (X), degenerated collagen fiber (Co) and calcified granules (arrowhead). E&F) T.E. micrograph of ligament showing presence of mast cell, macrophages (Mc), Lymphocyte (Lc), fibroblasts (Fc), degenerated collagen fiber (co).
III-2- ACL (ligament):
Light microscopy of the ligament showed variable degree of degenerative changes of the fibrous connective tissue faintly stained and containing few cells deeply stained surrounded by hallow zoon (Figure 5).
Transmission electron microscopy of the tissue forming ligament showed presence of fibroblasts having electron dens nucleus with presence of peripheral surface processes variable in its length and extended in homogenous light electron dens matrix. Surrounding these fibroblasts and matrix presence of bundles of collagen fiber of variable degree of electron density. Also, the presence of numerous electron dens granules variable in size in the surrounding tissue seem to be calcified deposit in degenerated cell organelles or fiber (Figure 6).
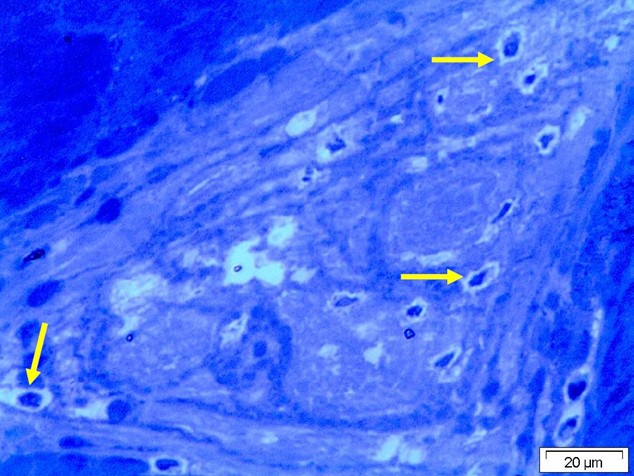
Figure 5: Light micrograph by Ziehl–Neelsen stain of tissue ligament showing degeneration of the collagen fiber with presence of deeply stained cells surrounded by hallow zoon or empty space (arrow).
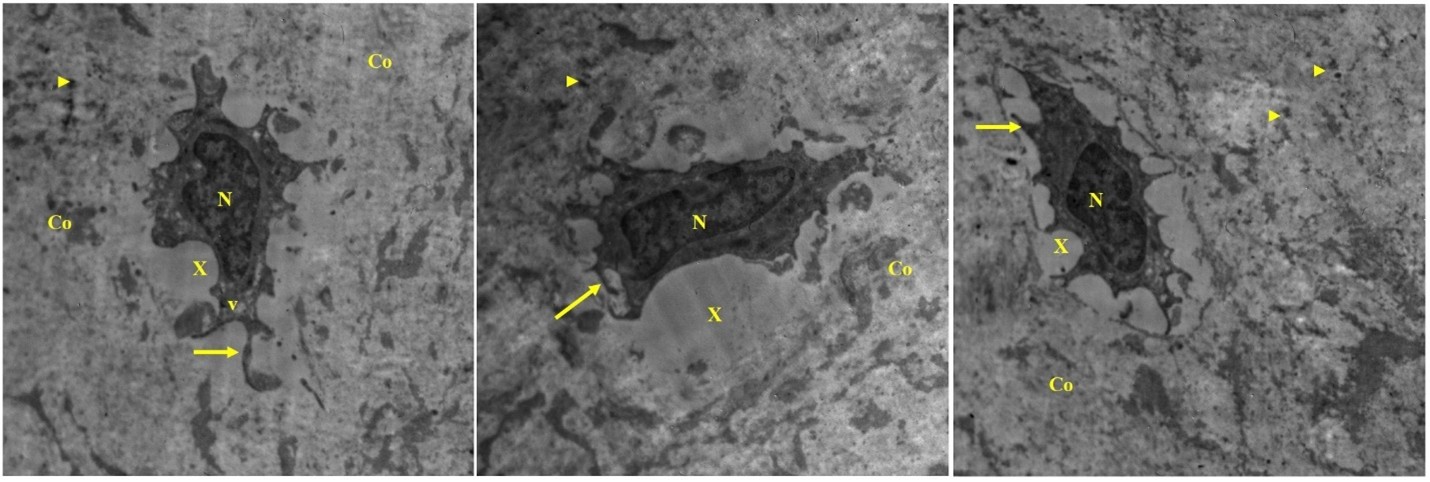
Figure 6: T.E. micrograph of the ligament showing fibroblasts embedded in light electron dens matrix (X), cells having electron dens nucleus (N), dilated vesicles (v) and surface processes (arrow), presence of electron dens calcified granules (arrowhead) in the degenerated collagen fiber (Co).
IV-ACL (Ligament):
Light microscopy of the ligament revealed the tissue forming the ligament contain bundle of collagen fiber in stat of variable degree of degenerative changes. The cellular component of the tissue is few and deeply stained surrounded by hallow zoon or empty space (Figure 7).
Transmission of electron microscopy of the ligament tissue was formed by collage fibrous tissue containing collagen fiber and fibroblasts. These cells have electron dense nucleus and cytoplasm with presence of surface processes of variable length extending to the surrounding matrix. Surrounding these cells, a considerable amount of light to moderate homogenous electron dens matrix having electron dens granules were observed (Figure 8). The collagen fiber of the ligaments showed variable degree of degeneration with the presence of numerous electron dens calcified granules. Some of these cells present in state of necrosis where the nucleus becomes small and condensed and the organelles lose its morphological structure, fragmented, and calcified (Figure 8).
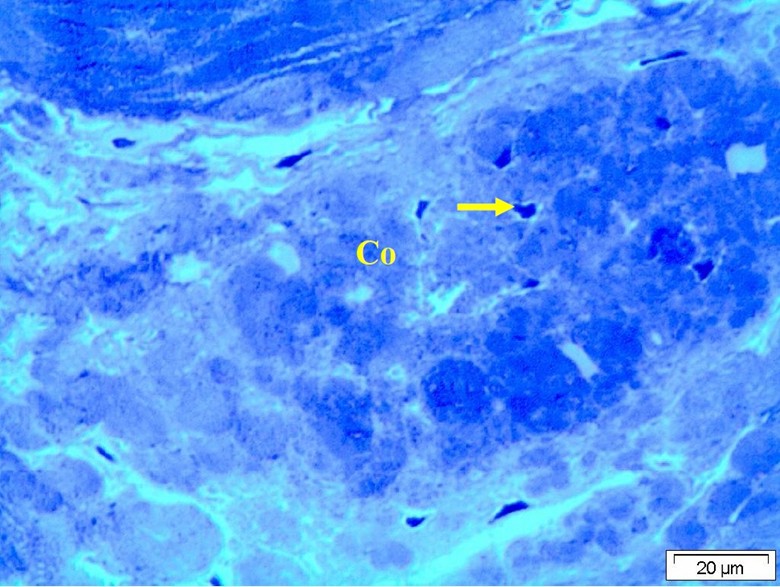
Figure 7: Light micrograph with T.B. stain of the ligament showing variable degree of degenerative changes of the collagen fiber (Co) and the cells appeared deeply stained surrounded by hallow zoon (arrow).
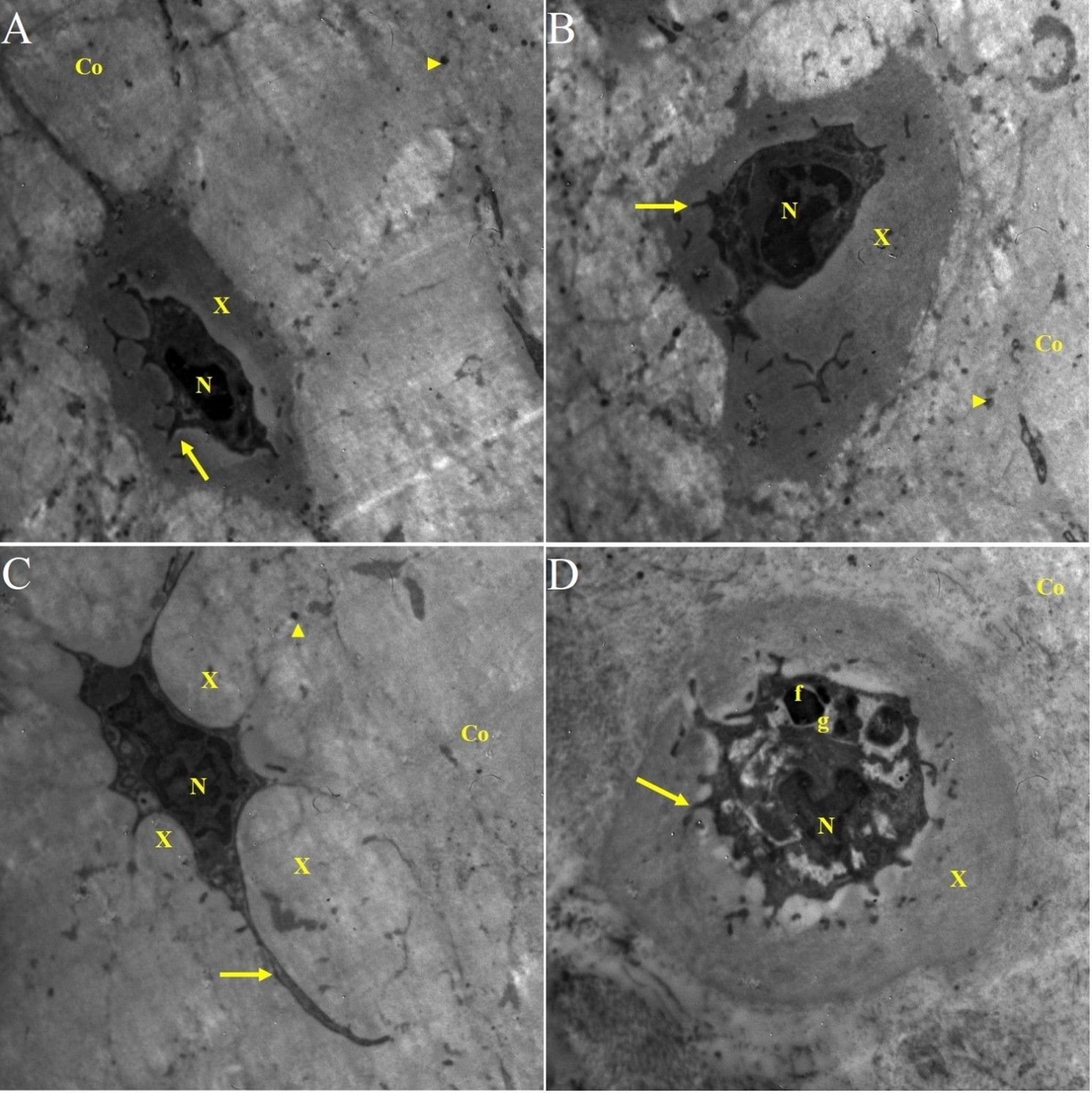
Figure 8: A&B) T.E. micrograph of the ligament showing presence of fibroblasts contain electron dens nucleus (N) and cytoplasm and embedded in moderate electron dens matrix (X). Notice presence of surface processes (arrow) and the surrounding collagen fiber (Co) degenerated contain calcified granules (arrowhead). C) T.E. micrograph of the ligament showing fibroblast contain electron dens nucleus (N), long surface processes (arrow) embedded in light electron dens matrix (x). D) T.E. micrograph of fibroblast of the ligament in state of necrosis showing condensed nucleus (N), fragmented cytoplasm containing electron dens bodies or granules (g) fat globule (f). Notice the cell embedded in moderate electron dens matrix (x) and surrounded by degenerated collagen fibers (Co).
Pathological Affection of ACL ligament
The pathological affection of the ligament was studied in four joints using both light microscopy and transmission electron microscopy. The light microscopy examination of the semi thin section of the ligaments revealed variable degrees of degenerative changes. These changes were in form of dysplasia of the fibroblast cells which appeared oval and mostly surrounded by hallow zone and fragmentation or disorganization of collagen fibers (Figure 1, 3, 5 and 7). Meanwhile, the affections of the ligament studied with transmission electron microscope revealed prominent pathological changes in the collagen fiber and fibroblast cells. The collagen fiber in some area which appeared somewhat normal formed by densely packed collagen fiber in random-packed network in little ground substances (Figure 2). The collagen fiber changes were in form degeneration or disorganization with increases of the ground substance of the tissue and presence of calcified granules varies in size and location in all examined sections (Figure 2, 4, 6 and 8). The tissue infiltrated with numerous mast cells, lymphocytes and macrophages (Figure 4). The fibroblast cells of the ligament revealed dramatic pathological changes include degenerative changes with the presence of fat globules (Figure 2), dilatation of the endoplasmic reticulum (ER) containing light electron dens material similar to that present in the ground matrix and that surrounding the fibroblasts (Figure 2, 4, 6 and 8). Numerous fibroblastic cells appeared dysplastic as a result of these morphological appearances such presence of surface processes, dilatation of the ER and embedded in matrix similar to the chondrocytes (Figure 2, 4, 6 and 8). Also, numerous fibroblastic cells found in state of necrobiosis or even necroses which appeared fragmented with calcification (Figure 2).
Discussion
The ACL provides structural integrity by contributing up to 85% of the total restraining force at 90° of knee flexion and 87% at 30° of flexion (13). In OA, inflammation is a major risk factor that can be associated with cartilage loss, join swelling and indicators of synovitis (14). Furthermore, several inflammatory factors were found to be associated with OA including tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β) and inflammatory cytokines. Other changes which can be found in OA patients that contribute to ACL inflammation manifest as leukocyte infiltration and neovascularization in the ligament sheath and within the ACL substance (9). Nawata et al. studied the changes in the ACL in collagen induced arthritis on rats. They found that the inflammatory changes affected the ACL greatly leading to ultimate failure load (15). Irie et al. reported that inflammatory cytokines appear immediately after an ACL injury and remain within the joint for one week (16). However, in chronic OA, inflammatory changes tend to stay for a prolonged duration. Hasegawa et al. studied osteoarthritic changes of the knee joints of 72-hour post-mortem cadavers and reported that there was a positive correlation between ACL changes and ACL degeneration (9).
In our study, inflammatory changes were found in the form of dysplasia of the fibroblast cells which appeared oval, mostly surrounded by hallow zone and fragmentation, or disorganization of collagen fibers. In addition, tissues were infiltrated with numerous mast cells, lymphocytes and macrophages. These inflammatory changes in our study confirm that ACL changes in the form of inflammatory infiltration and degeneration occur in OA patients which might contribute to the structural integrity of the knee joint and flexion. Therefore, treatments that target anti-inflammatory changes in the knee can show a better long-term effect such as platelet-rich-plasma (17).
Interestingly, morphological appearance such as the dilatation of the ER can be considered as a stress response (18). The ER is a protein-folding factory, responsible for the assembly and modification of numerous soluble proteins and membrane proteins. Newly synthesized protein translocate to the lumen of the ER where they are further processed and folded into three-dimensional structures. Therefore, when inflammatory protein cytokines starts forming and accumulate in the ER lumen it triggers a process known as ER stress (19). In chronic diseases such as arthritis, Alzheimer and diabetes, prolonged inflammation has been known to cause further cellular and morphological deterioration leading to more structural damage. Chavez-Valdez et al. studied the inflammatory effect on the ER of neurons after programmed death in animal samples. The ER stress was responsible for ER dilatation and mitochondrial swelling (18). Similarly, these ER changes were found in cardiovascular, atherosclerotic and metabolic diseases (20). Even though the ER stress is caused by the inflammatory changes induced by the disease, the prolonged disruption of the ER can cause what is called an ER stress pathway. This pathway further disrupts the metabolic functions causing inflammation. Therefore, the presence of ER dilatation in our samples can be attributed to the fact that the ER stress pathway is present in OA patients leading to further disruption of the inflammatory cytokines, ER dilatation and macrophage infiltration that were found in the ACL.
The study is limited by the number of samples taken. Moreover, the macroscopical changes of the ACL were not emphasized. More similar studies are required to understand the role of ER stress, ER stress pathways, inflammatory changes, and the role of ACL degeneration in OA patients.
Conclusion
The present study explores the microscopical changes of ACL in OA patients. Various microscopical inflammatory responses were found in the ACL ultrastructure. These include collagen fiber degeneration or disorganization, ER dilatation and macrophage infiltration. Such microscopical inflammatory changes can affect the ACL in OA and can lead to disruption of the knee structural integrity leading to further disruption of the process of knee OA. Studies on treatment modalities that target ER stress pathway are necessary to explore its role in regulation of OA and reduce the structural damage of the knee during the disease process and in aging.
Disclosure
Author contributions
The author has reviewed the final version to be published and agreed to be accountable for all aspects of the work.
Ethics statement
An informed written consent was obtained in both patients to be included in the study. An ethical approval from the institutional review board was granted to conduct the study by King Abdulaziz University Hospital.
Consent for publications
Not applicable.
Data availability
All data is provided within the manuscript.
Conflict of interest
The author declares no competing interest.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Acknowledgements
Acknowledgement goes to Dr Allam A.Nafady, professor of pathology and director of electronic microscopy unit in Assiut University, Egypt.