Volume 3, Issue 12
December 2023
Pathway of Infections and Virulence Factors in Endodontics
Ainaa Alsharif, Bassam Rawas, Rawan Zurayn, Reama Alaofi, Ahmed Alkhamis, Rahaf Halawi, Roua Khayat, Lama Khateri, Manahil Almutairi, Ammar Arab, Rfal Murshid
DOI: http://dx.doi.org/10.52533/JOHS.2023.31219
Keywords: Endodontic infections, apical periodontitis, microbial pathogenesis, virulence factors, root canal therapy
Endodontic treatment has increasingly become a preferred method for tooth preservation, largely due to its consistently high success rates. The success of endodontic therapy, aimed at tooth preservation, has improved due to a deeper understanding of endodontic pathology and advancements in treatment methods. Infections within the root canal, leading to apical periodontitis, are typically polymicrobial with a significant role played by obligate anaerobic bacteria. The study highlights the importance of understanding the intricate pathways of infection, such as through dentinal tubules, open cavities, the periodontal membrane, and the bloodstream, as well as the impact of faulty dental restorations. Key to managing these infections is an in-depth knowledge of the endodontic microbiota and its interplay with various disease states. The review also underscores the role of virulence factors in the pathogenesis of endodontic infections. These factors contribute to bacterial behavior, including adherence, invasion, evasion of host defenses, and induction of proinflammatory cytokines. Understanding these complex interactions is essential for developing effective treatment strategies and enhancing the field of endodontics, with a focus on maintaining long-term oral and overall health.
Introduction
Endodontic treatment has increasingly become a preferred method for tooth preservation largely due to its consistently high success rates. This surge in popularity and effectiveness is attributed to significant advancements in treatment techniques and a thorough understanding of endodontic pathology. The primary objective of endodontic therapy is to eradicate infections and prevent the re-entry of microorganisms in the root and adjacent periradicular tissues. Achieving this goal is highly dependent on a comprehensive grasp of the endodontic microbiota and its interplay with various disease states. Such knowledge is indispensable for the effective management and treatment of endodontic conditions, playing a critical role in ensuring the long-term health and maintenance of the affected teeth and their surrounding structures (1). Furthermore, the critical role of microorganisms in the onset and persistence of different types of apical periodontitis has been highlighted through extensive scientific research. This research has been pivotal in emphasizing the importance of microbial factors in endodontic diseases, leading to better treatment outcomes and an enhanced understanding of the etiology of these conditions (2).
Humans coexist with a diverse range of microbial species that form the normal or commensal microflora, often residing in biofilm communities on various mucosal surfaces such as the oral cavity, upper respiratory tract, gastrointestinal tract, urogenital tract, and skin. In healthy individuals, the absence of continuous inflammation suggests a balanced coexistence between these microorganisms and the body’s epithelial and mucosal surfaces. This balance is essential for bacterial survival without provoking harmful inflammatory responses (3, 4). Pathogenic microorganisms, capable of causing diseases in susceptible hosts, have the unique ability to adhere, colonize, survive, reproduce, and invade while avoiding the host's immune defences (5). Diseases and damage are more likely when these pathogens exhibit significant virulence, and the host's immune system is compromised. Therefore, understanding the dynamics between pathogenic organisms and the host's immune system is crucial in endodontics and broader medical sciences. The complexity of endodontic therapy lies in its role in maintaining both oral and overall health. It involves not just treating the immediate symptoms but also understanding the underlying causes and interactions between various microorganisms and the body's immune response. This comprehensive approach is vital for the successful treatment of endodontic diseases and for ensuring the longevity and health of the dental and periodontal structures (6). Endodontic therapy, therefore, is not just a dental procedure; it is an integral part of healthcare that requires an interdisciplinary understanding of microbiology, immunology, and pathology. By focusing on the elimination of infection and prevention of its recurrence, endodontic treatment plays a significant role in preserving natural teeth, thereby contributing to overall oral health and, by extension, to the general well-being of individuals.
Pathways of infection
Endodontic infection pathways are complex and multifaceted, playing a crucial role in the development and progression of dental diseases. The landmark study by Kakehashi et al. (2), has underscored the significant impact of microbiota in these infections, leading to a deeper understanding of how these microorganisms invade and affect the dental pulp (7).
One primary route of infection is through the dentinal tubules. These tubules can act as conduits for bacteria, particularly following a carious lesion or during dental procedures. The critical threshold for bacterial invasion in this case is when the distance of dentin between the carious lesion and the pulp narrows down to just 0.2 mm (8). This proximity allows bacteria to easily access and infect the pulp tissue.
Open cavities present another significant pathway for endodontic infections. Such cavities can occur due to traumatic events like coronal fractures or as a result of iatrogenic factors during dental operations. These incidents break down the natural barrier provided by dental structures, leaving the pulp exposed to the oral environment, which is teeming with potential pathogens (9). The periodontal membrane also serves as a pathway for microorganisms. Bacteria residing in the gingival sulcus can migrate to the pulp chamber through the periodontal membrane, utilizing lateral channels or the apical foramen. This route is particularly prevalent in circumstances such as dental prophylaxis, dental luxation, or as a consequence of periodontal pocket formation due to the downward movement of epithelial insertion.
Bloodstream infections represent another critical pathway. In cases of transient bacteremia, which are not uncommon in the daily lives of healthy individuals, bacteria can be attracted to the dental pulp, especially following trauma or procedures that cause inflammation without direct pulp exposure. This process, known as anachoresis, is a significant mechanism for the spread of endodontic infections.
Faulty dental restorations can also contribute to the spread of infection. Salivary contamination can reach the periapical area in under six weeks in canals sealed with guttapercha and sealer (10). If a temporary seal is compromised, the tooth structure fractures before final restoration, or if the final restoration is inadequate, bacteria can gain access to the periapical tissues, leading to infection. Lastly, the extent of infection can be influenced by the contiguous nature of dental tissues. Microorganisms might migrate from an infected tooth to a healthy pulp through the principal and/or lateral canals, thereby spreading the infection to adjacent teeth (9).
Virulence factors
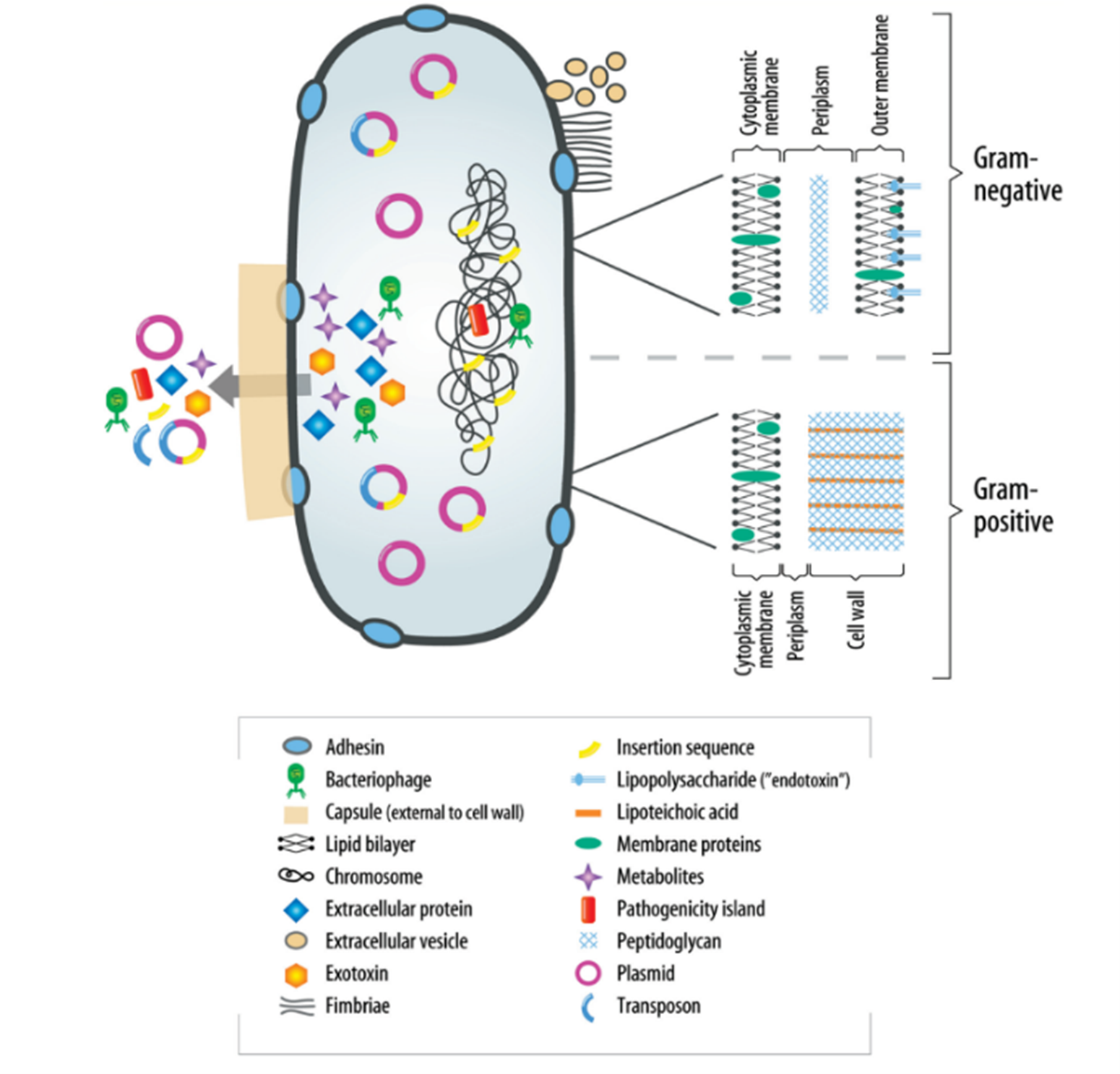
The understanding of virulence factors in endodontic infections is crucial yet still developing. These factors, diverse in nature, play a pivotal role in modulating bacterial behavior, including adherence, invasion, and evasion of host defense mechanisms. They can also cause host damage, both indirectly and directly. Although specific knowledge about these factors in relation to endodontic bacterial pathogens is limited at present, their importance cannot be overstated. Significant structural differences exist between gram-negative and gram-positive bacteria, particularly in their peripheral structures, as highlighted in (Figure 1) (6). These differences are critical to understanding how bacteria interact with their environment and host. Additionally, the periplasm of bacteria serves as a site for numerous vital processes and regulatory functions essential for cell viability and growth. It acts as a buffer against environmental changes, safeguarding the bacteria. A major aspect of bacterial virulence, particularly for many species, is the induction of proinflammatory cytokines, often stimulated by components of the bacterial cell wall.
Lipopolysaccharide
Lipopolysaccharide (LPS), also known as endotoxin, is a key component of the outer membrane in Gram-negative bacteria, consisting of lipid A, the LPS core, and the O-antigen polysaccharide side chain. This structure is crucial for bacterial environmental interactions, with lipid A anchoring LPS to the membrane and the core and O-antigen extending outward. The structural variation among bacterial species and strains affects their biological effects.
LPS activates the Hageman factor, triggering plasma protease cascades. Its lipid A diversity helps evade immune recognition, particularly by toll-like receptor 4 (TLR4) on immune cells (9, 11). This interaction stimulates proinflammatory cytokines' release. LPS upregulates vascular endothelial growth factor (VEGF) expression through TLR4 signaling (12). Additionally, LPS activates complement pathways and promotes IL-1β and IL-8 secretion in dental pulp cells (13). LPS also engages with a cluster of differentiation molecule 14 (CD14), enhancing inflammatory cytokine stimulation (14, 15). In endodontics, endotoxin's role in periapical bone destruction, pulpal pain, and inflammation has been extensively researched (16, 17). Higher LPS levels in symptomatic teeth highlight its importance in endodontic pathology (18).
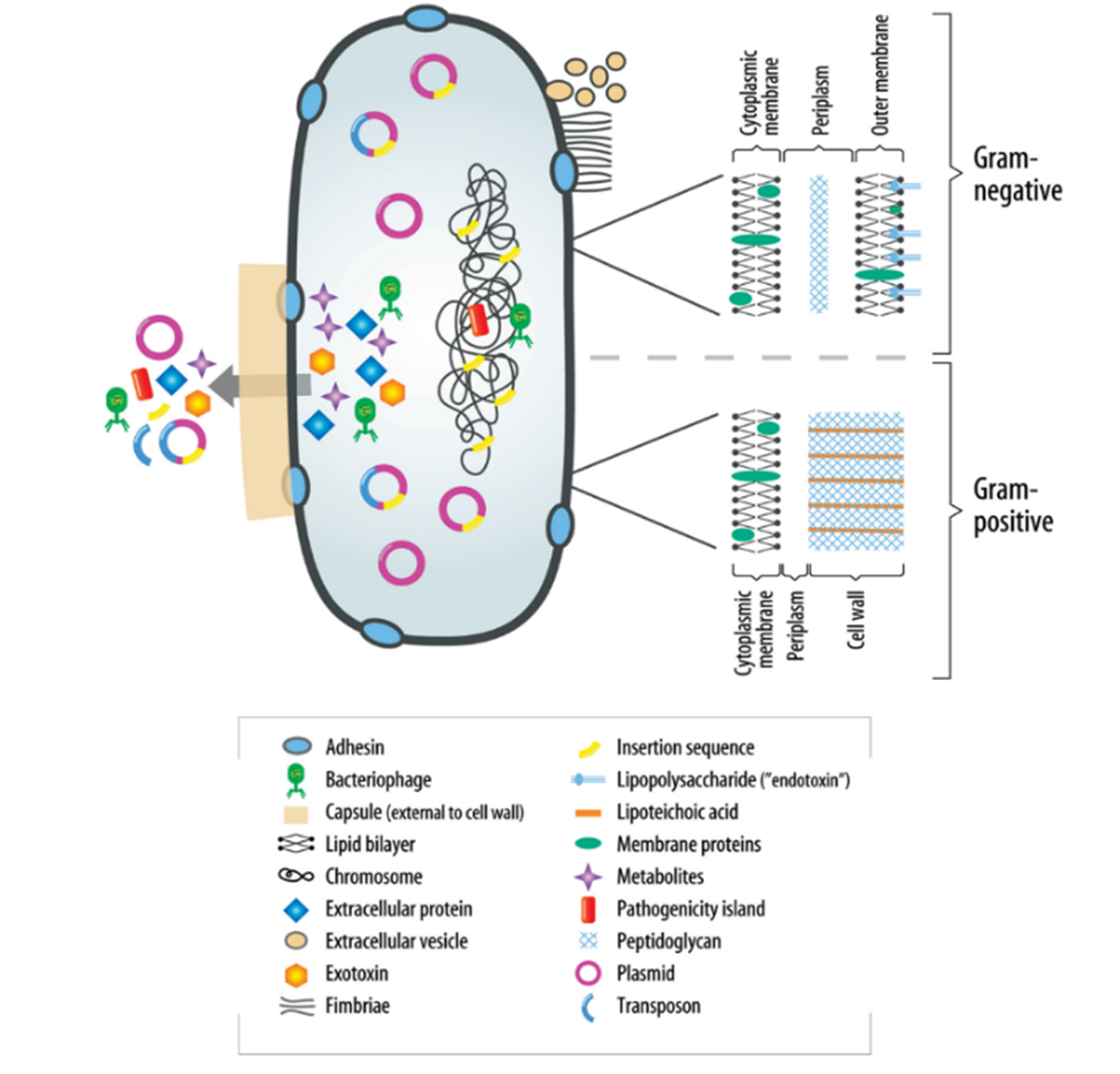
Figure 1: Virulence factors associated with bacteria (6).
Peptidoglycan
Peptidoglycan (PG) is crucial in maintaining the structural integrity and functionality of bacterial cell walls, especially in Gram-positive bacteria, where it shapes and strengthens the cell wall and manages cytoplasmic osmotic pressure. In Gram-negative bacteria, PG exists in a thinner layer. Released during cell lysis, PG significantly stimulates both proinflammatory and anti-inflammatory cytokines (9). PG's influence on the immune response is significant. For example, PG from Staphylococcus aureus induces interleukin-6 (IL-6) and IL-10 mRNA in monocytes and T cells, and stimulates IL-6 in human dental pulp cells (19). This cytokine expression is mediated through TLR2 in fibroblasts (9). Lactobacillus casei's PG also promotes IL-6 production in human dental pulp cells, depending on the time and dosage. Moreover, PG can aid in the adaptive immune response via macrophages (20).
The effectiveness of PG is notably increased when combined with LPS (19, 20). This synergy is particularly relevant in endodontic infections, which are often polymicrobial with a prominent presence of gram-negative anaerobes. Understanding the interplay between PG, LPS, and the host immune response is key to grasping the complexities of endodontic infection pathophysiology and developing effective treatments.
Lipoteichoic acids
Lipoteichoic acids (LTA) in gram-positive bacteria are significant in inducing inflammatory diseases, including nephritis and septic shock (9). LTAs interact with cell membranes and immune cells, influencing the expression of proinflammatory chemokines and cytokines (19, 21).
Fimbriae
Fimbriae, essential components of bacterial cell surfaces, play a pivotal role in the pathogenesis of various infections, including endodontic infections. These thin, filamentous macromolecules, composed of protein subunits, are typically up to 10 nanometers (nm) in diameter and range from approximately 100 nm to several micrometers in length. Unlike flagella, which are longer and involved in cell motility, fimbriae are primarily involved in the attachment of bacteria to surfaces and interactions with other bacteria. There are various types of fimbriae, each playing a distinct role in bacterial adherence and interaction. For instance, type 1 fimbriae of uropathogenic Escherichia coli (E. coli) facilitate adherence to urinary epithelium (22), while type IV fimbriae, found on Eikenella corrodens, can form bundles and have been detected in root canal samples from teeth with acute periapical abscesses (23).
Fimbriae's role in bacterial adherence and invasion varies among different types and species. For example, Prevotella intermedia fimbriae can induce hemagglutination (9) and the type II fimbriae of Prphyromonas gingivalis exhibit greater adherence to epithelial cells compared to type I fimbriae, thus potentially contributing more significantly to virulence (24).
Capsules
Bacterial capsules, primarily composed of polysaccharides, are crucial for protecting bacteria and evading host immune responses. These capsules enable bacteria to avoid opsonin-mediated phagocytosis and recognition by the complement system and antibodies, hindering subsequent phagocytosis (25). This is especially evident in gram-negative, black-pigmented bacteria, where capsules improve evasion from or survival after phagocytosis (9). Capsules also facilitate the movement of pathogenic strains within the host, as seen with Streptococcus pneumoniae (S. pneumoniae), where capsule formation allows efficient migration to epithelial surfaces, unlike in capsule-deficient mutants (26). Moreover, they protect microbes from environmental threats, including host defenses, desiccation, bacterial viruses, and toxic substances. In endodontic pathogens like Porphyromonas gingivalis (P. gingivalis), the capsule is a key virulence factor, aiding in evading phagocytosis and dampening the host's inflammatory response, thereby promoting bacterial survival (27). Understanding capsules is vital for comprehending how bacteria persist in hostile environments and elude immune defenses in endodontic infections.
Extracellular vesicles
Extracellular vesicles, originating from the outer membrane of gram-negative bacteria, represent a significant aspect of bacterial virulence and intercellular interactions. These vesicles, typically 50–250 nm in diameter, encapsulate proteins and lipids from the periplasm (9) and engage in various virulence-associated activities, including bacterial adhesion, proteolysis, hemagglutination, and hemolysis (28). They play a critical role in modulating interactions between bacteria, as evidenced by their ability to induce aggregation among various species like Streptococcus spp. and Fusobacterium nucleatum (9).
Moreover, extracellular vesicles can offer protection to the bacteria by binding substances like chlorhexidine. Certain virulence factors, such as leukotoxin from Agregatibacter actinomycetemcomitans (A. actinomycetemcomitans) and the arginine-specific and Lysine-specific gingipain from P. gingivalis, are associated with these vesicles, highlighting their role in enhancing bacterial pathogenicity. The detection of outer membrane vesicles in lesions associated with refractory apical periodontitis using transmission electron microscopy underscores their significance in endodontic pathology (9).
Exotoxins
Exotoxins are potent toxins released by bacteria that have a significant impact on eukaryotic cells and other microbes. They interfere with cell structures either by modifying actin or by influencing Rho guanosine triphosphatase regulators, which are essential for the immune system (29). Superantigens produced by Streptococcus pyogenes, which can lead to multiorgan failure, underscore the seriousness of exotoxin effects. In methicillin-resistant Staphylococcus aureus, toxins such as panton–valentine leukocidin and protein A combine to cause severe infections, including hemorrhagic necrotizing pneumonia (30). These superantigens are also implicated in periodontitis, contributing to an increase in proinflammatory cytokines (31).
Distinct from exotoxins, bacteriocins target other bacterial species. These protein or peptide-based substances either inhibit or destroy closely related bacterial strains, influencing microbial interactions in settings like infected root canals (9).
Extracellular proteins
Bacterial extracellular proteins, including various enzymes, play a vital role in the development and severity of endodontic infections. By interacting with TLRs, these proteins activate both innate and adaptive immune cells, leading to cytokine production. Their range includes histolytic enzymes like hyaluronate lyase and chondroitinsulfatase, which facilitate tissue disintegration. Extracellular proteinases, particularly from Bacillus pumilus in necrotic pulps and apical periodontitis, degrade collagen and elastin, worsening apical periodontitis (32). Post-treatment apical periodontitis has been linked to proteins from Streptococcus gordonii and Streptococcus anginosus (33). The collagenase gene in P. gingivalis is associated with larger periapical lesions. In E. faecalis, extracellular proteins like cytolysin, serine protease, and gelatinase are key. They enhance bacterial adherence to dental structures and survival in root-filled teeth, as found in many E. faecalis strains from infected root canals (34).
Metabolic by-products
Bacterial metabolic by-products significantly impact the pathophysiology of endodontic infections. These by-products, including reactive free radicals and superoxide anions, are released into the extracellular space by living bacteria or following cell lysis. Notably, certain bacteria, like E. faecalis, produce extracellular superoxide, which can lyse erythrocytes. E. faecalis's superoxide production also enhances its survival in mixed infections, as seen in its interaction with Bacteroides fragilis. Short-chain fatty acids, such as butyric and propionic acids, are fermentation by-products of obligate anaerobes. These acids stimulate cytokine release, influencing endodontic infection dynamics. For instance, butyric acid has been found to penetrate root canals in teeth filled with gutta-percha (9), hinting at its role in periapical infections. Overall, understanding the complex roles of these pathways of infection, and virulence factors is essential for comprehending the pathogenesis of endodontic infections and developing targeted treatment strategies.
Conclusion
Endodontic infections reveal a complex interplay of various microbial species, including bacteria, fungi, viruses, and archaea, contributing to the disease process. The success of endodontic therapy hinges on a deep understanding of these microorganisms and their unique pathogenic mechanisms. Knowledge of different infection pathways and the role of virulence factors is vital for developing effective treatments, ensuring long-term dental health, and advancing the field of endodontics.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.