Volume 3, Issue 8
August 2023
Birthmarks and Vascular Anomalies in Children: Classification, Diagnosis and Management
Abdulghani Alzamzami, Mansour Alzamanan, Abdullah Al Ali, Yazeed Alqazlan, Mohammed Al Bensaad, Fahad Al Munajjim, Reham Magharbel, Asma Asiri, Hamad Aldasem, Abdulwahab Alenezi, Asma Alshammari, Amroo Noorelahi
DOI: http://dx.doi.org/10.52533/JOHS.2023.30803
Keywords: vascular, anomalies, classification, children
Birthmarks are a common presentation among newborns and the majority do not require any medical intervention. However, vascular anomalies, which are an umbrella of diverse pathological conditions require special attention for a multitude of factors. Vascular anomalies lesions can appear at any stage of development, including prenatal, perinatal, and childhood. They are broadly classified as vascular malformations and vascular tumours. While many lesions have a straightforward natural course, more complex, extensive, or progressive lesions can pose a threat to life due to mass effects and severe complications including haemorrhage, or high-volume arteriovenous shunting. Infantile haemangioma, congenital haemangioma, and kaposiform hemangioendothelioma are the sub-classifications of vascular tumours, with infantile haemangioma being the most prevalent. Depending on the nature, location, extent, and accompanying manifestations of the tumour, there are several management options for vascular tumours, including conservative methods, pharmacological therapy, and surgical intervention. Low-flow and high-flow lesions are two subcategories of vascular malformations. Capillary, venous, and lymphatic malformations are examples of low-flow lesions, while arteriovenous malformations, a highly varied set of lesions that can present in a number of ways, are classified under high-flow lesions. Endovascular or surgical obliteration is the basis of treatment for these dynamic lesions. The purpose of this research is to review the available information about birthmarks and vascular anomalies in children; classification, diagnosis, and management.
Introduction
Dermatological skin manifestations that first reveal themselves within the first few days of life or are present from birth are categorized as neonatal cutaneous conditions. These cutaneous findings range from physiological and transient manifestations to serious pathological concerns and are mainly caused by the structural and functional growth of the skin. Most of these cutaneous abnormalities are benign; therefore, no intervention is necessary right away. However, some birthmarks and skin infections can be significant and need to be managed appropriately (1). Birthmarks are quite prevalent, and the majority of them are harmless. Some birthmarks frequently appear over the first few weeks or months of life; they are not always visible at birth. This is largely related to the development of newborn skin and the gradual deepening of skin tone. Over time, melanocytes' increased synthesis of pigment in the skin helps to distinguish between normal and abnormal hypo- and hyperpigmented skin abnormalities. Birthmarks might be either a standalone skin issue or a crucial diagnostic tool for other, more serious diseases (2).
Vascular birthmarks are observed in almost 20%–30% of newborns, which are typically incidental observations but can occasionally include progressed forms including salmon patch and nevus simplex. About 1% of neonates have capillary anomalies, while 4% of mature neonates have infantile haemangiomas (IH). Vascular anomalies are categorized based on the vascular type that is most noticeable in them (3). About 1 in 10 newborns are affected by vascular birthmarks every year. Birthmarks resulting from vascular anomalies can be categorized into distinct categories of vascular tumours and vascular malformations. Congenital haemangioma and IH are instances of vascular tumours, whereas capillary, lymphatic, venous, and arteriovenous malformations (AVM) are examples of vascular malformations or a combination of these. While some vascular tumours, like IH, eventually involute, vascular malformations typically endure and frequently worsen with time. Vascular anomalies may lead to major side effects such as persistent pain, deformity, recurring infections, or coagulopathy, necessitating the use of medication and/or surgery (4).
It has been noted that the majority of vascular birthmarks occur in an autosomal dominant way, and some of these birthmarks have been observed to exhibit inheritance patterns in families. This indicates that the majority of inherited vascular anomalies affect both sexes equally often, with a 50% chance that a child would receive the mutant allele (5). Vascular anomalies have a characteristic endothelium turnover, appearing at birth and developing in conjunction with the growth of the child. A precise diagnosis using contemporary diagnostic techniques that can distinguish between low-flow and high-flow lesions is essential for subsequent therapeutic management. Along with conservative treatment methods, other viable therapeutic options include laser usage, sclerotherapy, interventional embolization, and surgical intervention. Multidisciplinary care for patients is required for optimal management (6). The purpose of this research is to review the available information about birthmarks and vascular anomalies in children; classification, diagnosis and management.
Methodology
This study is based on a comprehensive literature search conducted on May 29, 2022, in the Web of Science, Medline, and Cochrane databases, utilizing the medical topic headings (MeSH) and a combination of all available related terms, according to the database. To prevent missing any possible research, a manual search for publications was conducted through Google Scholar, using the reference lists of the previously listed papers as a starting point. We looked for valuable information in papers that discussed information about birthmarks and vascular anomalies in children: classification, diagnosis, and management. There were no restrictions on date, language, participant age, or type of publication.
Discussion
Vascular birthmarks fall under the umbrella of congenital lesions that pose a considerable cosmetic and functional burden for patients. Due to their widely diverse clinical appearance and frequently intricate anatomical relationships, they continue to be a diagnostic and therapeutic challenge for clinicians. Accurate diagnosis is necessary for the best functional and cosmetic outcomes because each type of vascular lesion has a specific, tailored treatment plan. Uncomplicated lesions like lone haemangiomas and harmless capillary anomalies are easily diagnosed and treated by primary care physicians. Specialized multidisciplinary clinics are essential to the management of several other vascular birthmarks due to their complexity and relative rarity (7). Haemangiomas develop as a result of cellular division. Rapid neonatal growth is their defining feature. Spontaneous regression starts when a baby is 6 to 10 months old, although it can last up to 10 years. Rarely are haemangiomas life-threatening; however, pharmacologic therapy is essential, and surgical intervention is necessary for complicated cases. Vascular malformations are lifelong conditions characterized by dysplastic vessels. They are either fast-flowing abnormalities with arteriovenous shunting or slow-flowing anomalies. Additionally, complex mixed vascular abnormalities are seen. Angiographic examinations from ten years ago made the disparities between the various lesions’ crystal evident. Nowadays, a non-invasive diagnostic method is advised, especially for children. The most reliable techniques are magnetic resonance imaging (MRI), doppler flow imaging, and ultrasonography because they can show how much tissue is involved and can distinguish between fast-flow and slow-flow anomalies. Depending on the type of vascular malformation, risks, and therapy vary (8).
Classification and diagnosis
Haemangiomas and vascular malformations are two distinct types of vascular anomalies that Mulliken and Glowacki characterized in 1982 while proposing a biological categorization system. The International Society for the Study of Vascular Anomalies are most recent classification scheme was approved in 2014 and includes more complex syndromes with additional abnormalities, more causal genes for vascular anomalies, and a more detailed definition of vascular anomalies (9).
Haemangiomas
With a prevalence of 2%-3%, haemangiomas are the most prevalent tumour in infancy and the majority of proliferating vascular tumours. IH and congenital haemangiomas are two types of vascular tumours while congenital haemangiomas are further subdivided into non-involuting congenital haemangiomas (NICHs) and rapidly involuting congenital haemangiomas (RICHs) (10).
Congenital haemangioma
Approximately 30% of benign vascular tumours are congenital haemangiomas, which, unlike IH, are present at birth. RICHs achieve their maximum size by birth and involute within 12 to 18 months, but NICHs continue to increase in size according to the patient and do not involute. RICH lesions may usually be monitored with conservative treatment and only need to be treated when they are close to important structures like the orbits. The head and neck or the extremities are the most likely places for both RICHs and NICHs to present as single lesions. Clinical examinations are used to make the majority of haemangioma diagnoses. In contrast to IHs, NICHs, and RICHs have comparable clinical lesion morphology. NICHs typically appear as pink-to-purple elevated lesions with pronounced central or periphery telangiectasia and blue pallor (11, 12). RICH lesions typically appear as elevated, grey-blue lesions with core depression, ulceration, or scarring and significant telangiectasia (13).
IH
Almost 70% of all haemangiomas are IHs, which appear after birth. In 60% of cases, they are located at the head and neck, while 25% have been identified on the trunk, and 15% are located on the extremities. The majority of the time, they emerge within the first six weeks of life and exhibit a triphasic pattern of evolution, including proliferation, plateauing, and involution. The majority of IHs attain their maximum size by 3 to 5 months of age. Proliferation occurs during the first few months of life. The majority of IHs start involution at one year of age, with complete involution occurring in 50% of cases by five years, in 70% of cases by seven years, and in virtually all cases by 8 to 12 years of age. However, total involution does not imply total resolution. Up to 40% of IHs will still exhibit scarring and fibrofatty residuum-characterized skin alterations. Skin blanching, telangiectasia, and an erythematous macule are frequently the first symptoms of IHs. As the proliferating cells multiply, they frequently develop into elevated lesions that are bright red or strawberry in colour and have a plaque-like appearance, occasionally with centre ulceration (10). A typical presentation of IH is presented in Figure 1.
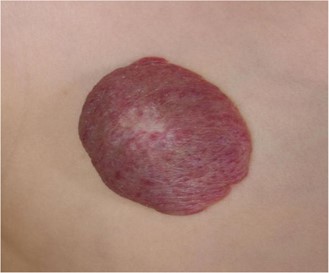
Figure 1: Typical presentation of IH (23)
Vascular malformations
Low-flow and high-flow lesions are two categories of vascular malformations. Capillary, venous, and lymphatic malformations (LM) are categorized as low-flow lesions. The AVMs are a highly varied collection of lesions that can exhibit in a number of ways and are classified under high-flow lesions (14).
Capillary malformations
Capillary malformations are post-capillary venule abnormalities that affect the head or neck and are low flow, sporadic, congenital, and persistent. The majority are asymptomatic, although laser therapy can be used to lessen skin thickness and discoloration. Less than 1% of children have Port Wine Stains (PWS), a significant form of capillary malformation with no sex predominance. Due to their significant associations with disorders like Sturge-Weber syndrome and Klippel-Trenaunay syndrome, these lesions should be distinguished from simple capillary malformations. PWS are congenitally present and increase and deepen in colour with age. Local soft tissue and bone enlargement cause variable degrees of deformity in PWS patients (14).
LM
When the primordial lymphatic channels fail to develop properly during embryonic development, they result in LMs, which are congenital anomalies made up of dilated lymphatic channels. They are filled with a protein-rich fluid, but they are not typically linked to the body's natural lymphatic system. Macrocystic, microcystic, or mixed lesions are possible. At birth or in early infancy, soft, nonpulsatile lumps with normal skin on top are lymphatic anomalies. Macrocystic lesions can be detected/identified during the late first trimester using prenatal ultrasonography. LMs that were not detected during pregnancy usually manifest at birth or before age two (15). The case of an axial LM is shown in Figure 2.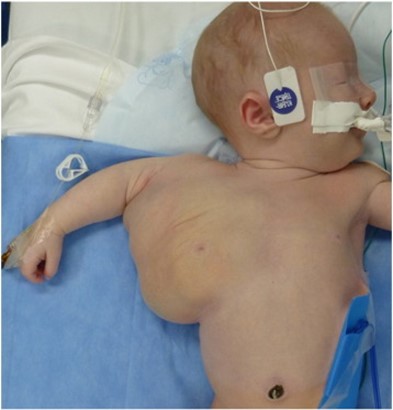
Figure 2: Case of axial lymphatic malformation (24)
Venous malformations (VM)
Up to 40% of all identified vascular anomalies are VMs. Similar to all birth defects, they have an equal impact on both genders. VMs can affect both soft tissue and bone and result in disability or disfigurement. Children typically have a soft, blue, fluctuant skin lesion on the mucous membrane or cutaneous skin surface. The lesion may swell, but related to the mass effect, venous congestion, and occasional painful thrombosis, chronic discomfort is more frequently present (14).
AVM
AVMs are dynamic, congenital vascular lesions that are rare. In the lack of a typical capillary bed, a nidus creates a direct connection between the arterial and venous systems, causing blood under arterial pressure to be shunted into the low-pressure venous system. The majority of AVMs are minor and asymptomatic; however, some of them grow during adolescence. Disfigurement, functional disruption, haemorrhage, and high-output heart failure can all be driven by growth and local invasion. The Schöbinger classification is used to stage clinical progression (14).
Vascular malformations, as opposed to vascular tumours, are abnormalities that involve malformed arteries without endothelial cell growth and occur while the vascular system is still developing morphologically. They are always there at birth, though some only become apparent later. Some vascular malformations are stable, whereas others may worsen as a child grows due to hormonal changes, trauma, or infections. Vascular anomalies are typically diagnosed clinically. However, in individuals with unusual illnesses, non-invasive imaging modalities, including computed tomography (CT), MRI, and ultrasonography, and perhaps even histological techniques, may be required to identify the nature and extent of the lesions (16).
Differentiating vascular anomalies or determining the degree of bone involvement and/or destruction can be done with the use of selective radiography imaging (17). Mittal et al. described that the primary imaging method used to diagnose vascular anomalies is sonography in conjunction with doppler imaging. Patients with these lesions are typically seen in a busy routine ultrasonography setting, or even in an emergency where a prompt and precise diagnosis is required. Even for radiologists with less experience, a straightforward algorithmic approach based on sonographic findings in conjunction with clinical aspects aids in obtaining a solid diagnosis of these lesions. Lesion extent and vascular supply assessments benefit from further imaging utilizing MRI or CT. However, it is impossible to overstate the value of a comprehensive history and examination in a case of a vascular anomaly since they serve as the foundation for distinction (18). A step-by-step diagnostic approach for vascular anomalies is illustrated in Figure 3.
Management
Treatment and management of vascular anomalies depend on their type and extent of severity. In order to effectively treat vascular anomalies, a collaborative approach involving paediatricians, plastic surgeons, interventional radiologists, paediatric surgeons, otorhinolaryngologists, and paediatric oncologists is necessary. In many centres, a variety of treatment modalities, including conservative treatment, surgical excision, injection sclerotherapy, cryotherapy, laser therapy, angiographic embolization, angioplasty/binding of feeder's vessels, the use of angiogenesis inhibitor drugs, and particularly the use of readily available corticosteroids, have produced positive results (19). Sainsbury et al. reported that, with acceptable morbidity and recurrence profiles, intralesional bleomycin injection effectively cured complex and recurrent vascular anomalies (20). Nowadays, laser therapy is vital for the treatment of paediatric vascular lesions. The thermocoagulation laser energy can be shaped to handle various lesions by finding the right balance of wavelength, energy density, and pulse duration. It is possible to deliver the best selective treatment with the minimum side effects (21).
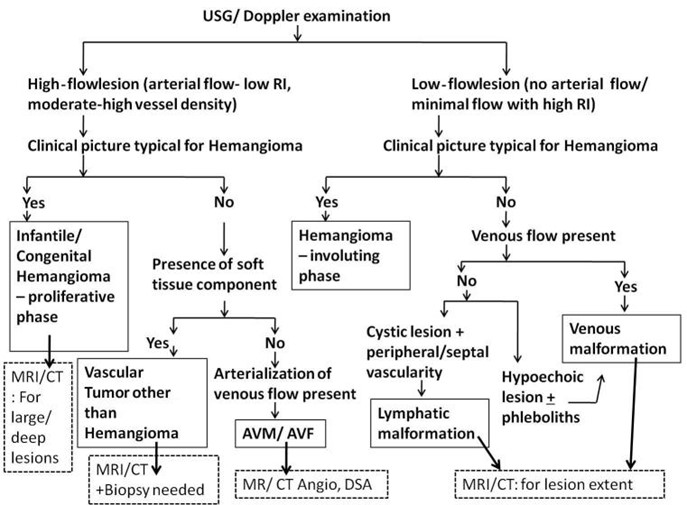
Figure 3: Flowchart for diagnostic approach of vascular anomalies in children (18)
*AVF: arteriovenous fistula ** AVM: arteriovenous malformation, ***CT: computed tomography; ****DSA: digital subtraction angiography; *****MRI: magnetic resonance imaging; ******RI: resistive index
Cox, Bartlett, and Lee narrated that the key to a successful course of treatment, like with any medical or surgical aberration, is a correct diagnosis. In general, invasive and non-invasive treatments are used to treat symptomatic lesions in vascular malformation care. The majority of capillary malformation therapy options, including pulsed-dye lasers, are ablative in nature. Initiation of early therapy leads to better outcomes; hence, it is advised that treatment begin before the child turns 6 months old. Surgery is only used for lesions that are resistant to ablative therapy or that are very disfiguring. Absolute ethanol sclerotherapy is helpful for treating extensive vascular malformations, but it should be used carefully because it might harm nerves, resulting in skin necrosis, and cause systemic toxicity. Bleomycin and 3% sodium tetradecyl sulfate are two additional commonly utilized sclerosants. Surgery is rarely used as a first-line treatment; however, it may be in some circumstances. The treatment options for LM include beginning with expectant therapy of symptomatic lesions, such as pain relief, compression for intralesional haemorrhage, and antibiotics for infection. The idea behind treating an AVM is to remove the nidus, which is assumed to be the source of the lesion's formation, by attracting new blood vessels from nearby areas. To enable safer intraoperative resection with minimal blood loss, sclerosing and embolizing procedures continue to be first-line alternatives (22). A comprehensive review of birthmarks and vascular anomalies among children is illustrated in this paper, and the literature elaborately explains this concept and its characteristic features; however, further clinical research can be beneficial in developing new and more effective treatment modalities and additionally aiding in studying their outcomes.
Conclusion
Birthmarks are widespread, and most require no clinical intervention. Vascular anomalies, on the other hand, need close monitoring for early diagnosis and prompt management. Preservation of functionality, reduction of related symptoms, and preservation of esthetic integrity are the objectives of vascular malformation management and treatment. Early intervention is necessary for severe functional impairment that poses a risk to life. Consequently, a multidisciplinary team is required to provide optimal treatment. Furthermore, all types of birthmarks shall be critically examined by a neonatologist to identify or detect any abnormality in time.
Disclosure
Conflict of interest
There is no conflict of interest
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.
