Volume 5, Issue 12
December 2025
The Impact of Genetic Variations on the Success Rates of Endodontic Treatments
Abdullah Saad Al-Humaidi , Hatim Mohammed Alhefdi, Fatemh Mousa Alhashem, Mohammed Abdullah Alqarni, Mahdi Mohammed Alwusaybie
DOI: http://dx.doi.org/10.52533/JOHS.2025.51208
Keywords: endodontic treatment, genetic variation, root canal system, matrix metalloproteinases
Endodontics focuses on the structure, function, diseases, and injuries of the dental pulp, along with the health and treatment of the periodontium, particularly regarding apical periodontitis caused by pulp infection. Endodontic treatment involves the chemical and mechanical preparation of root canals to facilitate the placement of biocompatible obturating materials. The primary goals are to eliminate microorganisms and organic debris that foster bacterial growth and to restore periodontal health. Treatment success is typically evaluated after one year, based on criteria such as symptom resolution, absence of sinus tracts, and normal radiographic findings. If lesions persist after this timeframe, outcomes are deemed uncertain, and retreatment is often advised after four years, per the European Society of Endodontology. Histological success is defined by the healing of periradicular tissues and the absence of inflammatory cells, with overall success rates ranging from 31% to 96%. Microorganisms within the root canals significantly undermine treatment outcomes, leading to pulp infection and persistent symptoms. Factors influencing success include broken instruments, anatomical variations in the tooth, and inadequate canal cleaning. Notably, success rates vary by tooth type, with three-canal teeth (90%) exhibiting higher success than two-canal (80%) and single-canal teeth (70%). Importantly, genetic variations also influence endodontic treatment outcomes by affecting disease susceptibility, progression, and therapeutic responses. Genetic predispositions can result in conditions such as persistent apical periodontitis. For instance, genes responsible for matrix metalloproteinases (MMPs) expression, which are involved in inflammation and bone remodeling, significantly impact the progression of periapical periodontitis and may alter treatment efficacy. Higher levels of MMPs may correlate with poor healing outcomes, underscoring the need for further research into the role of genetic factors in endodontics. This review aims to highlight the importance of genetic considerations in treatment efficacy and to advocate for additional scholarly investigation into this emerging field.
Introduction
Endodontics studies the form, function, diseases, and injuries of the dental pulp. Additionally, it studies the periodontium and its health, prevention, and treatment, since apical periodontitis is primarily caused due to pulp infection (1). Endodontic treatment is a combination of chemical and mechanical preparation of the root canals to facilitate the placement of a biocompatible material to obturate the canals. The objective of endodontic treatment is to remove microorganisms and organic material that decompose due to the presence of microorganisms and harbor bacterial growth, in addition to restoring the health of the periodontium (2). Preparing the root canals aims to remove the non-vital or inflamed pulp to disinfect the root canals and resolve symptoms, and restore apical periodontal health (1).
The quality of the root canal treatment is assessed after at least one year based on certain criteria that include complete resolution of pain and other symptoms, absence of sinus tracts, no functional loss, and normal periodontal ligament space around the roots in the radiography (1). However, if a lesion did not resolve completely after one year or remained the same, then the outcome of the endodontic treatment is considered uncertain (1). The European Society of Endodontology recommends assessing the lesion and the apical periodontal health after four years, and if the lesion persists, then retreatment is recommended (1). Histologically, the success of endodontic treatment is assessed by the complete healing of the periradicular tissues and the absence of inflammatory cells (3). The success rate of endodontic treatment ranges between 31% and 96% and 85.2% based on the radiological findings. However, it is challenging to assess the success rate of endodontic treatment clinically, since other factors contribute to the success of the treatment outcome (4, 5).
The presence of microorganisms in the root canals is the primary factor that compromises the success of endodontic treatment. This is attributed to the role of microorganisms in causing pulp infection, inflammation, necrosis, and periradicular infection (2). Additionally, the incomplete removal of microorganisms causes continued inflammation, infection, and persistent symptoms (2). Other factors that affect the success of endodontic treatment include broken files, the presence of a foreign body in the periarticular tissues, perforation, and missed canals (2). Additionally, the type of the treated tooth, the pre-operative condition of the pulp, a previously endodontically treated tooth, overfilling of the canals or the access cavity, constant trauma to the tooth, and the presence of an idiopathic lesion contribute to the decrease in the success of the treatment and lead to failure in some cases (2). The type of tooth can affect the endodontic outcome. Although molars have a complex endodontic system, the success rate in three-canals teeth (90%) is higher than in two-canals teeth (80%), whose success rate is higher than that of single-canal teeth (70%). This hypothesis states that the narrow canals in multirooted teeth allow near-optimum mechanical and chemical preparation of the canals compared to single canal teeth that possess very large canals, which renders optimum mechanical and chemical preparation challenging (2).
Despite the presence of several factors that directly affect the success of endodontic treatment outcomes, genetic factors play a crucial role in determining such outcomes. Genetic variation is the dental anatomy of teeth that directly affects the degree of susceptibility to diseases, the progression of the diseases, the response to the treatment, and therapeutic outcomes. Genetic variations contribute to the inflammatory response, cellular signaling, enamel, dentin, pulp, and cementum formation, which can either facilitate the endodontic treatment or render it challenging (6).
This review article seeks to elucidate the influence of genetic variations on the efficacy of endodontic treatment, emphasizing the significance of genetic factors in determining treatment outcomes and their long-term implications. By addressing a notable gap in the existing literature, this article advocates for further scholarly investigation into this innovative and pertinent area of research.
Methodology
This narrative review is based on a comprehensive literature search conducted on August 1, 2025, using ScienceDirect, PubMed, Wiley Library, Dynamed, MDPI, Oxford Academic, BMC, and Cochrane databases. The research utilized Medical Subject Headings (MeSH) terms and relevant keywords, such as Genetic variation and its effect on the outcomes of endodontic treatment, to identify studies that examined dental genetics and analyzed their effects on the success rate of endodontic treatments. A manual search was also conducted using Google Scholar, and the reference lists of identified papers were reviewed to locate additional relevant studies. No restrictions were applied regarding publication date, language, participant age, or type of publication, ensuring a broad and inclusive exploration of the available literature.
Discussion
Although the root canal system is complex and completely disinfecting it is challenging, endodontic treatments have high success rates ranging between 85% and 95% (7). Endodontic treatment has the highest success rates when the pulp is vital. This is attributed to the presence of microorganisms in the pulp chamber only (7). However, failure can be attributed to the recontamination of the root canal system or the persistence of the infection, especially in the periradicular area, which leads to root and bone resorption (7). The success rate of endodontic treatment can be influenced by the presence of a hermetic seal, the absence of spontaneous and provoked painful symptoms, repair of periodontal tissue, and restoration of function (7). The most prevalent reason for the failure of endodontic treatment is the reinfection or persistence of infection due to a lack of a coronal seal (8, 9). Therefore, endodontic treatment clinical and radiographic follow-up is mandatory to ensure longevity of the treatment (Figures 1 and 2) (10). The European Society of Endodontics recommends taking a follow-up radiograph after one year, and in case of a periapical lesion, radiographs should be taken two to four years post-operative (11, 12).
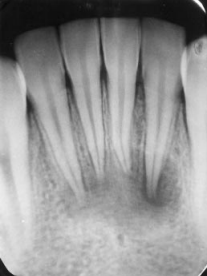
Figure 1: A radiographic image of lower incisors with large periapical lesions (13).

Figure 2: A radiographic image of a 3-month follow-up post-endodontic treatment depicting healing of the periapical lesions (13).
Multiple host-related factors affect the success rate of the endodontic treatment, such as age, gender, systemic disorders, and the immune system (14). The cortisol hormone plays an essential role during the chemo-mechanical preparation of the root canal. It is secreted in males more than in females; therefore, flare-ups and failures are noted in females more than in males (15). Additionally, patients with systemic diseases who receive steroids are less likely to experience flare-ups post-endodontic treatments (15). Patients who are 50 or older were found to experience flare-ups eight times higher than younger patients (16). This is attributed to the changes in humoral and cell-mediated immune responses in older individuals. However, the changes of the pulp tissues in the elderly, such as decreased vascularity, contribute to better endodontic outcomes and higher success rates (16).
Human genetic variants are classified as either common or rare. Common variants, also known as polymorphisms, have a minor allele frequency (mAF) of at least 1% in the population, while rare variants have an mAF of less than 1% (17). Additionally, genetic variants can be categorized by their nucleotide composition into two main classes: single-nucleotide variants and structural variants. SNPs are the most prevalent type of genetic variation found among individuals. It is estimated that the human genome contains at least 11 million SNPs, with approximately 7 million having a mAF of over 5%, while the rest fall within the range of 1% to 5% (17). On the other hand, structural variants include any base pairs that differ between individuals and are not classified as single-nucleotide variants. This category encompasses a range of variations, including insertions and deletions (indels), block substitutions, and inversions of DNA sequences (17). These genetic variations can directly affect the inflammatory response of an individual to microorganisms, such as the interleukin 1 inflammatory mediators, which play a crucial role in both humoral and cell-mediated inflammatory responses (18).
Genetic predisposition in certain genes can cause persistent apical periodontitis. Compounds, such as matrix metalloproteinases (MMPs), play a significant role in inflammation, bone remodeling, and bone resorption (19). MMPs stimulate osteoclastic bone resorption. They can link native, nondenatured collagens with long, uninterrupted triple helices and act as collagenases (20). MMPs degrade the organic matrix and are stimulated by bone-resorbing cytokines, such as IL-1 and IL-6. They play a critical role during the progression of periapical periodontitis. Additionally, successful root canal treatment could interfere with their expression. If their levels are elevated, failure of lesion healing is predicted (21).
Genetic predisposition in the MMP genes can significantly affect the level of bone destruction and remodeling and further exacerbate periapical lesions in teeth with deep carious lesions. Menezes-Silva et al. (22) experimented on individuals with several deep carious lesions that did not result in periapical lesions and compared them to individuals with deep carious lesions with periapical lesions measuring more than 3 mm to assess the influence of genetic variation in MMP genes on the progression of carious lesions in dentin and the development of periapical lesions. They found a significant correlation between MMP2 and MMP3 and the presence of large periapical lesions, suggesting that MMPs may be associated with periapical lesion formation due to untreated long-standing deep carious lesions, resulting in bone remodeling and inflammation in response to deep caries (22).
Endodontic treatments tend to fail due to reinfection or persistent infection of the root canal system, which acts as a reservoir for microorganisms, resulting in apical periodontitis (23). Such conditions stimulate the release of proinflammatory cytokines, which stimulate pulpal tissue destruction by regulating MMPs (24). As a result, MMPs cause tissue breakdown in the periapical area, whereas MMP-3 breaks down the extracellular matrix (25). Therefore, variants in MMP genes result in faster progression of deep carious lesions in dentin and periapical pathologies (22).
Furthermore, genetic variations within proinflammatory genes, particularly allele 2 of the interleukin-1 beta (IL-1β) gene, have been shown to exacerbate complex multifactorial diseases, including periapical periodontitis. This suggests that the presence of a specific genotype within the IL-1β gene cluster is associated with an increased prevalence of clinical signs and symptoms following endodontic treatment (26). The relationship between these genetic polymorphisms and the pathophysiology of periapical periodontitis indicates that individuals with certain genetic predispositions may exhibit a more pronounced immunoinflammatory response when confronted with bacterial accumulations (26). Such genetic variations can significantly influence the inflammatory milieu, leading to an exaggerated response during the progression of periapical periodontitis. In particular, the IL-1β polymorphism is known to enhance the production of interleukin-1, a crucial cytokine involved in the inflammatory response. This elevation in IL-1 levels has the potential to perpetuate the inflammatory processes within the periapical region, extending the duration and severity of inflammation even after the completion of endodontic therapy. Therefore, understanding the impact of IL-1β genetic polymorphisms on the inflammatory response could play a vital role in predicting treatment outcomes and improving patient management strategies in the context of periapical periodontitis (26).
Additionally, wound healing is affected by the upregulation and downregulation of the expression of multiple genes. Wound healing following endodontic periapical surgery represents a multifaceted biological process characterized by a dynamic interplay between cellular components and their surrounding microenvironment, alongside the host's physiological response to surgical intervention. The culmination of wound repair and regeneration is achieved through the development of vascular and functional extracellular matrices, ultimately leading to the structural remodeling of periapical tissues (27). The structural remodeling of these tissues is regulated by a complex network of genes that govern the upregulation and downregulation of stem cells, thereby facilitating the restoration of the anatomical and functional integrity of the damaged tissues (27). The wound healing process encompasses a multitude of factors, including the expression of extracellular matrix components, chemokines, cytokines, growth factors, remodeling enzymes, cellular adhesion molecules, and various wound healing-associated genes such as COL1A1, VTN, IL-4, TNF, and CTGF. These genes represent critical phases of wound healing, including the inflammatory, proliferative, and remodeling stages (27). The pathways of genes implicated in periapical wound healing illustrate the baseline expression of key wound healing marker genes, COL1A1, VTN, ITGA5, IL-4, TNF, ANGPT1, VEGFA, and CTGF, in periapical granulomas subsequent to surgical endodontic treatment. Ahmed et al. (27) suggested that successful periapical wound healing correlates with a reduction in the expression of mediators associated with the inflammatory phase (TNF), angiogenesis (ANGPT1), and the remodeling phase (CTGF).
The response of dental pulp to nociceptive stimuli exhibits considerable variability among individuals, influenced by a constellation of factors including sex, psychosocial dynamics, genetic predispositions, and the presence of chronic pain conditions. Mladenovic et al. (28) found that the rs165774 COMT polymorphism significantly contributes to variability in electric sensitivity responses. The presence of polymorphisms within the COMT gene has been implicated in pain sensitivity modulation, operating through catecholaminergic and opioid mechanisms (28, 29). Moreover, they found associations between the (GG) genotype of the intronic rs165774 COMT polymorphism and the threshold pain response to electric stimuli. Notably, carriers of the A allele for the SNP rs165774 have previously been correlated with reduced pain sensitivity within female cohorts (28). The literature indicates that among 22 different COMT polymorphisms investigated, the rs165774 variant provided the most significant association with sensitivity to cutaneous heat pain (30). However, Mladenovic et al. (28) found no association between the rs165774 COMT variant and the intensity of cold pain responses, which can be attributed to the differential mechanisms of pain transmission, particularly the distinct pathways involved in the sensation of thermal stimuli as opposed to electric stimuli (28). The difference noticed in pulp sensitivity between electric stimuli and thermal stimuli is primarily due to physical alterations in dentin rather than direct activation of nerve endings (31). The findings underscore the critical role that genetic variations play in influencing the overall success of endodontic treatment. These genetic differences have a profound impact across various stages of the treatment process, including pre-operative, intra-operative, and post-operative phases. Understanding these genetic factors is essential for the optimization of treatment outcomes, as they can affect patient responses to interventions, healing processes, and potential complications. Thus, the integration of genetic analysis into endodontic procedures may offer valuable insights and lead to the development of more tailored and effective treatment strategies for individuals undergoing such dental interventions. Ultimately, acknowledging and investigating these genetic influences could enhance clinical practices and improve patient care in the realm of endodontics.
Conclusion
The success of endodontic treatment is influenced by a multitude of factors, with genetic variations playing a significant role. These variations directly impact the host's cellular, humoral, and inflammatory responses to the microorganisms present within the root canal system. Such findings underscore the necessity for personalized approaches to endodontic treatment, highlighting the importance of understanding the unique physiological condition of each patient, particularly in the post-treatment healing phase. This review article identifies a notable gap in the existing literature and calls for further research in this critical area to enhance treatment outcomes.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding.
Ethical consideration
Non applicable.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.
