Volume 5, Issue 11
November 2025
Visual Field Defects in Early Indicators of Intracranial Lesions
Ahmed Thabit Alnahdi, Abdulaziz Ali Sagr, Abdulaziz Omar Alsehemi, Abdullatif Obaid Altowairqi, Abdulaziz Ibrahim Alshehri, Faisal Nasser M Alahmari , Haneen Raheem Jandeel, Qutov Tareq Alkhaldi
DOI: http://dx.doi.org/10.52533/JOHS.2025.51111
Keywords: Intracranial lesions, visual field defects, visual pathway, pituitary adenoma, craniopharyngioma, early diagnosis
Intracranial lesions are tumors that develop within the skull. They account for almost 2% of all types of tumors. However, intracranial lesions remain a persistent burden despite having a low incidence, given their high morbidity and mortality rates. Growing evidence continues to report an association between brain lesions and visual field (VF) defects. It has been proven that specific visual field defects can be caused by the presence of a tumor in a particular location in the skull, as it interferes with the visual pathway. Thus, visual field defects can act as signs to identify and locate certain intracranial tumors. Moreover, recent studies suggest that therapeutic approaches can influence visual field outcomes as well. Nevertheless, further research is required to merely understand the complex association between VF defects and brain lesions. In addition to integrating ophthalmic diagnostic tools with imaging techniques, the implication of deep learning tools and virtual reality offers great prospects for the early diagnosis and management of brain lesions. Most importantly, clinicians and ophthalmologists should be aware of this association, as VF defects might be the earliest signs indicating the presence of an intracranial tumor. This review aims to demonstrate the underlying mechanisms behind the association between intracranial lesions and visual field defects as well as the role of VF defects in the early diagnosis and management of brain lesions. Moreover, it showcases the latest advancements in cancer and ophthalmological diagnostics, particularly the use of deep learning and virtual reality to prompt the early detection, monitoring, and management of intracranial tumors.
Introduction
Intracranial lesions are those that develop within structures inside the skull. They can either arise directly from the brain tissue or within the intracranial space, like the pituitary gland, or be metastatic neoplasms (1). Whereas brain and other CNS tumors account for almost 2% of all cancer types(2)They still pose a challenging global burden. This is caused by the high rates of mortality and detrimental effects, where intracranial tumor patients have a 5-year survival rate below 20% (3). The rate is particularly higher in developed countries. This is attributable to advancements in diagnosis and screening techniques compared to those in developing countries (4). Brain tumors are the most prevalent among other intracranial tumors, accounting for 70% of all CNS cases. It is followed by embryonal tumors (42.8%), tumors of the neuroepithelial tissue (34.7%), and meningioma (24.1%) (5).
Intracranial tumors often interfere with afferent (sensory) or efferent (motor) visual pathways. Hence, they may initially manifest with ophthalmic symptoms. Each type of visual defect is specific to one type of intracranial tumor, depending on the tumor’s location. Each intracranial tumor tends to produce certain neuro-ophthalmic signs that can help identify and localize the lesion. Thus, recognizing the manifesting ophthalmic condition along with the associated visual pathway could help in the early diagnosis of brain lesions (1). These ophthalmic conditions vary in severity from low visual acuity to complete blindness, depending on the location and size of the lesion (6).
For instance, tumors involved with the chiasm, like pituitary adenomas, result in bitemporal hemianopia; tumors involved with the occipital lobe result in double vision, causing macular sparing; and those associated with the optic nerve may cause complete vision loss. Of greater concern, some patients may develop cranial nerve paralysis and diplopia as the tumor extends into the parasellar region (7). Given the high specificity of the visual field associated with each tumor based on its location, they offer great prospects for early diagnosis of brain lesions. Accordingly, clinicians and ophthalmologists should be aware of this association between different types of brain lesions and visual field defects, taking into account that such signs might be the earliest indication for the presence of a tumor, forming the basis for establishing a clear diagnosis of a brain lesion(8).
With the growing advancement in technology, deep learning has been successfully employed in various fields of medicine. Recently, it has been implicated in the field of ophthalmology, where it demonstrated the ability to predict potential visual field defects, aiding in the establishment of an accurate early diagnosis of VF defects. This review aims to explore the different visual field defects associated with different types of brain tumors and demonstrate the underlying mechanisms behind these associations. Moreover, it seeks to examine the diagnostic implications of these visual field defects in the early detection of intracranial lesions, as well as provide novel tools like deep learning and virtual reality to aid in establishing an accurate early diagnosis of intracranial lesions (9).
Methodology
This review is based on a comprehensive literature search including the most recent studies from 2015 to 2025 in the PubMed and Clinical Key databases, as well as Google Scholar. Utilizing MeSH (Medical Subject Headings) and relevant keywords, such as "intracranial lesions," "visual field defects," "visual pathway," "pituitary adenoma," "craniopharyngioma," "early diagnosis," and "optic pathway gliomas (OPG)," the search aimed to explore studies examining the visual field defects associated with different types of brain tumors and demonstrate the mechanisms behind these associations. Moreover, it seeks to examine the diagnostic implications of these visual field defects in the early detection of intracranial lesions. The search was not restricted by publication date, language, or type of publication to ensure a broad exploration of the available literature
Discussion
Normal Visual Pathway and Common Defects
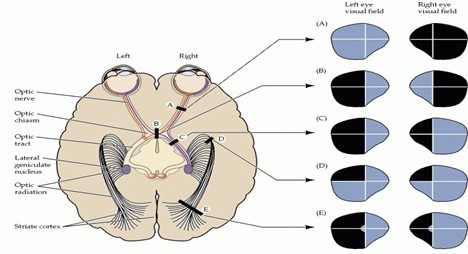
Understanding the normal functioning of the visual pathway is the first step in exploring the effects of intracranial lesions on the visual field. First, retinal photoreceptors detect light and then transform it into electrical signals. Such signals are processed by intermediate retinal cells and transferred to ganglion cells, the axons of which form the optic nerve. The optic nerves from both eyes extend posteriorly into the optic chiasm. At the optic chiasm, fibers from the nasal retina cross over. In contrast, temporal fibers remain uncrossed, allowing the left hemisphere to process the right half of the visual field and vice versa. Then the signals are transferred through the optic tracts to reach the lateral geniculate nucleus in the thalamus; information is further transferred through the temporal and parietal lobes. Finally, it reaches the occipital lobe, where visual images are perceived (10). On the contrary, the development of intracranial tumors causes compression, infiltration, and elevated intracranial pressure (ICP), prompting various visual field defects based on their location.
Among the most common visual field defects caused by intracranial lesions are homonymous hemianopsia, which is vision loss in the left or right eye, and bitemporal (heteronomous) hemianopsia, which is the loss of peripheral vision in both eyes. Left homonymous hemianopsia (which results in vision loss in the right side of the visual field), left superior quadrantanopsia (where the top left of the visual field is missing), and finally homonymous hemianopsia with macular sparing, in which vision is lost in the left side of the visual field while the central vision is preserved (11) (Figure 1).
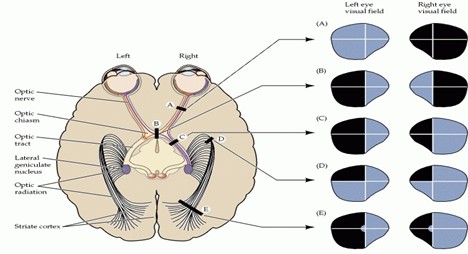
Figure 1: Tumor Types, Mechanisms, and Associated VF Defects
Pituitary Adenomas
Pituitary adenomas are a group of benign neuroendocrine tumors within the anterior lobe of the pituitary gland (12, 13). They are highly prevalent, accounting for 15–20% of all intracranial cases. They can either be functioning (secreting hormones such as lactotrophs, corticotropin, corticotrophs, somatotrophs, thyrotrophs, and gonadotrophs) or nonfunctioning. The latter is asymptomatic, which urges the need for enhanced diagnostics (14).
Pituitary adenomas arise in the sellar and suprasellar regions. Hence, they compress the optic chiasm as they increase in mass. This compression disrupts the visual pathway, hindering the transmission of signals through the nasal retinal fibers. Moreover, recent evidence by Chen et al. (2023) suggests that this chiasmatic compression results in microvascular changes that enhance ischemic injury and axonal degeneration, further exacerbating dysfunction (14, 15).
Bitemporal quadrantanopia is the most prevalent visual defect associated with pituitary adenomas. Ogra et al. (2014) reported that bitemporal quadrantanopia is the earliest visual field defect, given that the tumor grows upwards, affecting the upper temporal quadrants of both eyes. As a result, the tumor compresses the nasal fibers of the optic chiasm (16). Furthermore, asymmetric hemianopic loss could occur depending on the growth pattern of the tumor, as reported by Chen et al. (2023) in a study correlating microvascular changes in pituitary adenomas with VF defects; they also found that central scotoma or paracentral defects seldom occur if the optic nerve is directly compressed by the tumor, which leads to blurred central vision (15).
Craniopharyngiomas
Craniopharyngiomas are rare benign tumors with an incidence ranging between 0.5% and 2%. They arise from embryonic remnants and develop in the suprasellar region near the chiasm and hypothalamus, disrupting the visual pathway (17). They are predominant among the younger population, presenting within the first two decades. Nonetheless, a multicenter study in Spain by Iglesias et al. (2021) reported that such tumors occur in older adults, but the symptoms are often masked by the deterioration in health owing to senescence (18). The influence of the growing tumor on the chiasm and optic nerves compresses nasal fibers in the retina and leads to the development of bitemporal defects. As the tumor grows, it can extend to the optic nerve, resulting in central scotomas and eventually complete vision loss (19).
Similar to pituitary adenomas, bitemporal hemianopia is the hallmark VF defect caused by compression of the chiasm (17). Nevertheless, the unequal growth of tumors may cause asymmetric hemianopia. Given that the tumor extends further to reach the optic nerve, central scotomas develop, which may further progress into complete visual loss. A case study by Wang et al. (2020), including 7 malignant cases, demonstrated the progressive deterioration of vision despite enhanced therapy (20). Samanci et al. (2023) further authenticated this finding by using Gamma Knife radiosurgery to eliminate residual tumors. However, the associated VF defects showed no improvement (21).
Optic Pathway Gliomas (OPG)
Optic pathway gliomas are low-grade polycystic astrocytes (the most abundant type of glial cells responsible for supporting and nourishing surrounding neurons) (1). They are often implicated with the optic nerve, chiasm, or optic tracts or may extend to the hypothalamic region. In addition to causing progressive damage to axon and ganglion cells through direct infiltration of the visual pathway, they can also damage retinal fibers by compressing the chiasm and optic nerve. These lesions are more frequent in children suffering from von Recklinghausen disease, particularly neurofibromatosis type 1 (NF1), which is a genetic disorder characterized by alterations in skin pigmentation and nerve tissue caused by mutations in the NF1 gene. This, as reported by Bennebroek (2021; 2025), increases susceptibility to tumor development, where almost 50% of OPG cases are NF1 patients. He further demonstrated in a recent nationwide cohort study that, despite being more responsive to radiotherapy, NF1-associated gliomas have adverse VF outcomes and may lead to persistent visual field defects (22, 23).
The visual outcome depends on the location of the OPG. For instance, orbital tumors result in exophthalmos, which often presents with strabismus in 94% of patients and causes early vision loss in 88% of patients. If the tumor is in the optic chiasm or hypothalamic region, it may cause abnormal pupillary response, dyschromatopsia, optic atrophy, swelling of the optic disc, nystagmus, and elevated intraocular pressure. Owing to hypothalamic involvement, patients often present with endocrine insufficiency leading to disruptions in sexual maturation and growth caused by inhibition of growth hormone secretion. However, NF1-associated tumors often progress asymptomatically (24).
Meningiomas of the Planum Sphenoidale and Tuberculum Sella
Meningiomas are benign, slow-growing lesions originating from the meninges surrounding the brain and spinal cord (1). . Among the risk factors of meningiomas are NF2 gene mutations along with several environmental and lifestyle factors. They are the most prevalent among brain tumors (25). Clinical outcomes vary depending on their location; some patients may be asymptomatic, some may show endocrine or neurological abnormalities, while others manifest as visual field defects.
Lesions located in the Planum Sphenoidale and Tuberculum Sella cause visual field defects, including asymmetrical bitemporal hemianopia and junctional scotoma, that may lead to complete visual loss. Moreover, lesions in the olfactory groove may also affect vision if they are of a large enough mass(26). A neuroophthalmological examination can aid in diagnosis, where visual field testing identifies the extent of optic pathway involvement. Both fundus examination and optical coherence tomography of the retinal nerve fiber layer and macular ganglion cell complex will aid in determining prognosis after treatment of the tumor (27).
A case report by Salehipour et al. (2024) reported the dual diagnosis of a 42-year-old female with both. She presented with a headache and visual impairment. They demonstrated that there were only 7 similar cases reported with no common genetic basis or risk factors. This illustrates the importance of conducting additional research to confirm any potential correlation between them beyond mere coincidence (23).
Ophthalmic Implications and Early Diagnosis of Brain Lesions
Given the significant association of brain lesions with the visual field, VF testing offers a primary, noninvasive, and accurate diagnostic tool for detecting the location and type of such lesions. This is particularly essential for patients suffering from unexplained progressive visual field impairment. Growing evidence has proven VF defects as the earliest signs of the presence of brain lesions (Table 1). However, pediatric patients are often diagnosed late owing to overlooked symptoms; thus, setting structured, regular ophthalmological testing visits is required (6, 28).
In addition to tumor detection, visual field monitoring prior to, during, and after tumor therapy is essential, as treatment approaches have been reported to influence the patients’ visual field, particularly in cases of gliomas and adenomas. For instance, in a nationwide cohort study, Bennebroek, C. A. M., et al. (2025) reported visual field defects in 90% of pediatric patients after diverse treatments for pediatric OPG. Of greater concern, 20% of patients suffered from long?term binocular severe VI or blindness during the 3 years after treatment (22).
The combination of visual field detection tools like perimetry, fundus examination, and optical coherence tomography with imaging techniques like MRI and CT offers improved accuracy and reliability in diagnosis (29). The recent advancement in technology and the employment of deep learning in ophthalmological diagnostics offer great prospects for the early detection and management of brain lesions, along with the associated visual field defects.
For instance, Molina-Botello, D. et al. (2025) used MRI-based tractography, ultrasound, and VR mapping during parietal glioma resection involving optic radiations to improve tumor elimination and intraoperative monitoring and alleviate postoperative visual pathway complications (30). Furthermore, David, J. P. F., et al. (2025) developed an AI-based model for interpreting and reporting standard automated perimetry results. This model achieved 80% sensitivity and 94.64% specificity in detecting alterations in the visual field. Therefore, the employment of artificial intelligence promotes accuracy and reliability (31).
|
Table 1: Brain Lesions and Associated Visual Field Defects |
||||
|
Tumor |
Location in the Brain |
Associated VF Defects |
Method of Detection |
Author/Year |
|
Pituitary Adenoma |
Sellar and suprasellar region (compressing the optic chiasm) |
- Bitemporal quadrantanopia (earliest) |
- Visual field (VF) testing (perimetry) |
Ogra et al., 2014 (16); Chen et al., 2023(15) |
|
Craniopharyngioma |
Suprasellar region near optic chiasm and hypothalamus |
- Bitemporal hemianopia (hallmark) |
- VF testing |
Nuijts et al., 2020 (17); Iglesias et al., 2021 (18); Wang et al., 2020 (20); Samanci et al., 2023 (21) |
|
Optic Pathway Glioma (OPG) |
Optic nerve, optic chiasm, optic tracts, hypothalamic region |
- Early vision loss (often unilateral if optic nerve) |
- VF testing |
Bennebroek et al., 2021; Bennebroek et al., 2025; (22, 23) Modrzejewska et al., 2023(32) |
|
Meningioma (Planum Sphenoidale, Tuberculum Sella, Parasellar) |
Parasellar region, compressing optic chiasm/nerve |
- Asymmetrical bitemporal hemianopia |
- VF testing |
Salehipour et al. 2024; Baldassarre et al., 2025 |
Conclusion
In conclusion, having low survival rates, intracranial tumors remain a persistent issue despite their low incidence. Growing evidence highlights the significant neuro-ophthalmic association between brain lesions and visual field defects. VF defects are often the earliest indicators of not merely the presence of a brain tumor but also of its location and potential therapeutic outcomes as well. However, integrating visual field detection into routine tumor diagnostics requires further research and collaboration between various fields of medicine to explore this association. Furthermore, the employment of AI offers tremendous help in early detection and management of brain lesions and associated visual field defects. The current advancements and growing evidence urge the need for further research to better understand and utilize this association for improved diagnostic and treatment outcomes
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding.
Ethical consideration
Non applicable.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.