Volume 5, Issue 11
November 2025
The Impact of Imaging on the Early Detection of Breast Cancer Recurrence
Doha Ahmad, Saad Alqwaidi, Moath Althuneyyan, Ola Malawi, Amal Albuqaisi, Salem Alenezi, Abdullah Almutairi, Shuruq Al-Ayyafi
DOI: http://dx.doi.org/10.52533/JOHS.2025.51107
Keywords: MRI, Breast cancer, PET, PET/CT, Ultrasound, Mammography
Breast cancer remains the third most prevalent cancer globally, with approximately 1,960,681 cases diagnosed annually in females, contributing significantly to morbidity and mortality in both sexes. Recurrence is notably prevalent in women with estrogen receptor (ER)-positive and human epidermal growth factor receptor 2 (HER2) receptor-negative tumors, often occurring several years post-diagnosis. The presence of dormant micrometastatic cells, which can be reactivated when the immune response is compromised, accounts for a substantial recurrence risk. Despite these challenges, advancements in early detection and screening have markedly improved survival rates. The World Health Organization defines early detection as diagnosing symptomatic cancers at an early stage and early screening as identifying asymptomatic tumors. Alarmingly, a significant proportion of cases in regions such as Latin America and Sub-Saharan Africa are diagnosed at advanced stages, primarily by self-detection, leading to reduced prognosis. Imaging modalities play a critical role in post-treatment surveillance and early detection of recurrence. Techniques such as positron emission tomography-computed tomography (PET-CT), diffusion-weighted MRI (MR-DWI), and dynamic contrast-enhanced MRI (DCE-MRI) are pivotal for identifying metastases when conventional imaging fails. Corroboratively, histopathological assessments of steroid hormone receptors and HER-2 status are essential for confirming recurrences. The National Comprehensive Cancer Network (NCCN) endorses routine annual mammography for asymptomatic individuals’ post-breast-conserving therapy or mastectomy. Conversely, symptomatic patients should undergo advanced imaging techniques like CT, PET, and bone scans to evaluate potential recurrence. Furthermore, high-risk groups necessitate closer surveillance due to elevated recurrence probabilities, underscoring the importance of comprehensive imaging strategies in improving patient outcomes. Routine screenings using these tools can lead to early detection of tumors, resulting in better prognoses and the potential for complete resolution. To effectively identify recurrent breast cancer, a multidisciplinary approach is essential. This includes educating patients about the importance of regular screenings, annual imaging, and consistent physical examinations by clinicians. This review article aims to discuss the significant impact of different imaging modalities in the early detection of recurrent breast cancer.
Introduction
Breast cancer is marked as the third most common cancer in the world, with an estimated global incidence of breast cancer increasing to 1,960,681 cases in 2017 (1). It's considered the leading cause of death due to cancer in females. Additionally, breast cancer can cause death in males as well, accounting for 181,004 deaths annually. Its effect extends to causing disability, accounting for 17.7 million disability-adjusted life years (1, 2). Breast cancer mortality rates are high in both developing and non-developing countries, which renders it a global burden (1). The recurrence of breast cancer can occur up to 20 years after primary diagnosis. For instance, a patient who was previously treated with breast-conserving surgery was found to have 15% local recurrence and 21% of distant metastasis 20 years after treatment (3). Additionally, in recurrent tumors, lymph node metastasis and tumor grade are often higher, and the tumor size is often larger (4).
Despite the high recurrence rate of breast cancer, it has high survival rates, which is attributable to several factors, such as early-stage detection and screening (1). The World Health Organization (WHO) defined early detection of breast cancer as the diagnosis of a symptomatic cancer at an early stage, whereas it defined early screening as the identification of an asymptomatic cancer in a healthy population at an early stage (5). The majority of the advanced recurrent tumors are detected by the patients before being referred to health centers. If the diagnosis of breast cancer is delayed for more than three months, the prognosis and the survival rate are significantly decreased, which highlights the importance of early detection and screening (6).
These increased risks of recurrence after primary treatment necessitated post-treatment surveillance. The main purpose of post-treatment surveillance is to detect recurrent tumors early, when curative intervention is feasible (7). Early detection and screening significantly improve survival rates. It has increased the rates of survival, with an estimated 3.1 million women in the United States alone. The high recurrence rate of breast cancer as a second tumor in the same breast or in the contralateral breast requires surveillance and routine screening to detect it in an early stage, prompting an increased survival rate and a better outcome, hence improving the patients’ quality of life (8). However, despite the different routine screening protocols, the majority of recurrent tumors (71.5% of the distant metastasis and 56% of ipsilateral locoregional recurrent tumors) are detected outside the scheduled surveillance. Moreover, the recurrent tumors that were detected outside the scheduled surveillance were mostly prevalent in younger patients, patients with the HER2-positive breast cancer subtype, and patients with lymph node-positive breast cancer (9). These challenges in the post-treatment surveillance have shifted the screening routines to more advanced imaging modalities.
Mammography is the most common imaging modality used for post-treatment surveillance in breast cancer. It enables the early detection of asymptomatic recurrent breast cancer tumors. Mammography detects about 50% to 80% of ipsilateral breast recurrences and about 45% to 90% of contralateral recurrences (7). Another imaging modality that is considered an adjunctive tool to mammography is ultrasonography. It is easy to perform, has no radiation hazards, is inexpensive, does not require a contrast agent, and enables biopsy under guidance. It detects about 88% of contralateral recurrences, among which 43% are indetectable using mammography (7). Breast magnetic resonance imaging is a supplementary tool that enables recurrence detection due to its high specificity and sensitivity (7). Additionally, Positron emission tomography/CT is very useful in staging recurrences detected on physical examination (7).
This review article seeks to elucidate the significant role of various imaging modalities in the early detection of breast cancer following initial treatment. By examining the advancements and applications of these imaging techniques, the article aims to highlight their contributions to improving patient outcomes and enhancing the efficacy of post-treatment surveillance.
Methodology
This narrative review is based on a comprehensive literature search conducted on August 18, 2025, using ScienceDirect, PubMed, Wiley Library, Dynamed, MDPI, Oxford Academic, BMC, and Cochrane databases. The research utilized Medical Subject Headings (MeSH) terms and relevant keywords, such as imaging effect on the early detection of breast cancer recurrence, to identify studies that examined the influence of different imaging modalities on the early detection of recurrent breast cancer. A manual search was also conducted using Google Scholar, and the reference lists of identified papers were reviewed to locate additional relevant studies. No restrictions were applied regarding publication date, language, participant age, or type of publication, ensuring a broad exploration of the available literature.
Discussion
Breast Cancer Recurrence
Breast cancer recurrence has different types, including local recurrence, regional recurrence, and distant metastasis. Local recurrence is a recurrence in the original tumor location with the same histopathologic characteristics (10). Whereas regional recurrence is a metastasis of the disease in areas such as internal mammary nodes, infraclavicular nodes, ipsilateral axillary nodes, or supraclavicular nodes, with or without the involvement of ipsilateral breast tissues (10). Distant metastasis is the recurrence of the tumor in a distant location from the primary tumor site (10, 11). Several risk factors cause breast cancer recurrence, including the steroid hormone receptor status, the intrinsic biological subtype, the Nottingham tumor grade, and the Nottingham prognostic index (12). Such risk factors urge routine screening and imaging of patients at high risk for breast cancer recurrence, to decrease the morbidity and mortality rate. For instance, the subtype Luminal A often tends to metastasize to the bone and the liver. Davey and Kast et al. (13, 14) analysed about 2000 breast cancer patients and found that the subtype of the breast cancer plays a crucial role in predicting metastasis.
Role of Imaging in Detecting Breast Cancer Recurrence
In cases in which metastasis is suspected, imaging is mandatory. Examinations include physical examination, hematology, and biochemistry tests, and imaging of the chest, bones, and abdomen (15). Other imaging modalities, such as positron emission tomography–computed tomography scans (PET–CT), diffusion-weighted magnetic resonance imaging (MR-DWI), and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), can detect the location of metastases or suspected lesions when routine imaging tools fail to do so (15). Additionally, histopathological tests are required to confirm the diagnosis of recurrence, whether it is local or distant metastasis. Further evaluation of the steroid hormone receptors and HER-2 status is required as well (15).
The National Comprehensive Cancer Network (NCCN) has developed guidelines and recommendations for routine screening to detect breast cancer recurrence. The recommendations include an annual mammography for the surveillance of treated breast cancer patients, which is considered the cornerstone of surveillance in breast cancer (16). The annual mammography is specifically recommended for asymptomatic individuals who have completed breast-conserving therapy or mastectomy (16). However, symptomatic patients are recommended to undergo CT, PET, and bone scans to determine any recurrence or metastasis (17). Women who are considered at high risk of recurrence, such as women with a family history, dense breasts, or benign lesions, should undergo supplemental imaging, such as MRI, ultrasound, and mammography (17).
Imaging Modalities in Early Detection of Recurrent Breast Cancer
Ultrasound and MRI are significantly effective in the early detection of breast cancer recurrence, accounting for 95.1% and 100%, respectively (18). Although the NCCN has recommended annual mammography, it was found to be effective in the detection of breast cancer recurrence, with only 78.7% (18). The utilization of diverse imaging modalities for post-treatment follow-ups in breast cancer patients is critically important, given the significant likelihood of tumor recurrence irrespective of the surgical intervention or treatment regimen employed. For instance, a retrospective study conducted by Kurt et al. (18) revealed that 82.8% of the cohort presented with invasive carcinoma as their primary tumor, while 75% of the same cohort experienced invasive carcinoma upon recurrence.
Clinicians often require an MRI to confirm the presence of a regional recurrence after their physical examination and to follow up on the integrity of the implants. Kurt et al. (18) found in their study that physical examination was negative in 59.4%, which highlights the importance of MRI, since physical examination could not detect tumors of size less than 1 cm. On the other hand, mammography is better at detecting lesions in the early stage with better prognosis, yielding mammography-detected lesions with high survival rates (19). However, mammography sensitivity and specificity in women with breast cancer are lower than those in healthy women or women with no history of breast cancer. Moreover, its imaging of lesions often tends to present in a malignant state, regardless of whether the lesion is malignant or benign (20). The drawbacks of mammography in certain aspects advocate for the use of ultrasound, since it is more effective in detecting recurrence in the chest wall and axillary area (21). Additionally, ultrasound can differentiate between the changes that occur after mastectomy and local recurrence. Therefore, using ultrasound in follow-ups and detecting local and regional recurrence can be more efficient than using mammography (22). In comparison, MRI is more efficient in detecting primary cancer than ultrasound and mammography; however, the role of MRI in detecting recurrent tumors is still limited. It was found to have high sensitivity and specificity in detecting recurrence one year post-operative (23). However, a study conducted by Park et al. (24) reviewed 1000 MRIs of breast cancer patients and concluded that MRI is most efficient in detecting recurrence after three years post-operative.
Positron Emission Tomography (PET) is an imaging technique that takes advantage of the high glucose consumption of malignant cells by measuring the uptake of 18F-FDG, a glucose derivative (25). 18F-FDG is transported into tumor cells through the cell membranes by glucose transporters Glut-1 and Glut-3, and it is then phosphorylated by hexokinase. Factors such as inflammation and reduced oxygen levels also increase the retention of 18F-FDG in tumor cells (25). PET has high specificity in detecting breast cancer, ranging between 71% and 95% and high sensitivity, ranging between 80% and 90% (25). PET was found to be twice as sensitive as mammography and ultrasound combined in terms of detecting breast cancer (25). However, its sensitivity decreases significantly when detecting some tumors, due to their small size, metabolic activity, subtype, growth pattern, and proliferation rate (26). On the contrary, the sensitivity and specificity of PET are not affected by tumor cell percentage, lymph node status, histopathological grade, steroid receptor status, the presence of inflammatory cells, or GLUT-1 receptor expression (26). When comparing the efficiency of PET with MRI in detecting primary tumors, no significant difference was found. However, PET is highly accurate in detecting axillary nodal metastasis, with sensitivity ranging between 79% and 100% and specificity ranging between 66% and 100%. PET sensitivity in detecting ductal carcinoma was about 66% whereas its sensitivity in detecting lobular carcinoma was about 25% (27). This difference in sensitivity resulted in arguing that PET is insufficient to diagnose breast cancer, in addition to its high cost. However, it was shown that PET was significantly accurate in detecting local recurrent breast cancer and distant metastasis (Figure 1), accounting for 89% to 98% accuracy. Additionally, PET is more accurate than mammography and CT in detecting mediastinal and internal mammary nodes involvement (25).
To further increase the specificity and sensitivity of PET, researchers suggested combining CT and PET. FDG-PET/CT is used to estimate the therapeutic response of the tumor, detect recurrence or distant metastasis, and detect tumors in the early stage. Additionally, FDG-PET/CT can locate metastasis in unexpected locations (28). A prospective evaluation conducted by Shawky et al. (28) found that PET/CT was able to detect contralateral lesions in two patients (Figure 2), which traditional CT imaging could not detect. Moreover, PET/CT imaging could detect lesions in the bone marrow which was not detected by CT imaging as well (28). Several studies have shown that PET/CT can detect additional lesions than those detected by CT, and it can detect unsuspected distant metastasis in just one whole body scan (29, 30). Bone metastasis is the most common site of breast cancer metastasis. Both CT and PET/CT can detect osteoblastic, osteoclastic, and mixed bone metastases with the same sensitivity and the same specificity (28). However, PET/CT can detect bone metastasis earlier than CT and can detect bone infiltration that cannot be seen with traditional CT imaging. Additionally, it can detect the resolution of bone infiltration before the appearance of definitive signs of healing (28).
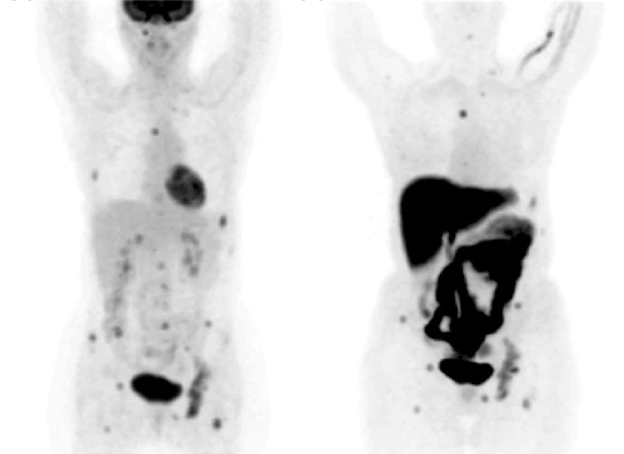
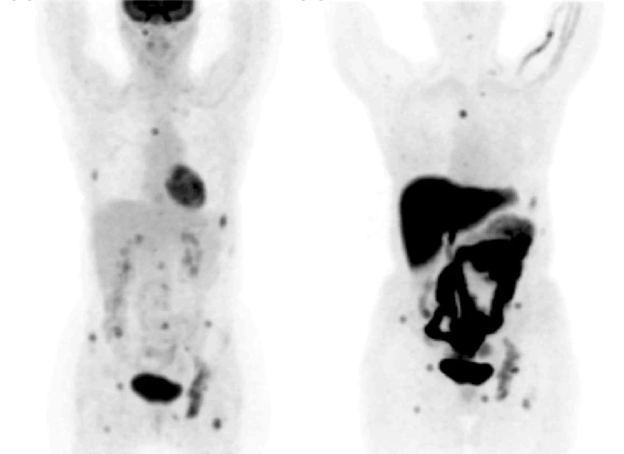
Figure 1: PET showing recurrent breast cancer metastasis in the bone (25).
The liver is the most common visceral organ to which breast cancer metastasizes. When recurrent local or regional breast cancer occurs, accompanied by liver metastasis, the survival rate significantly decreases (28). PET/CT could detect local and regional recurrent lesions with hepatic metastasis early. These lesions were of a size less than 3 cm, which highlights the significant ability of this imaging modality to detect small lesions in comparison with PET, which could not detect small lesions (28, 31).
Figure 2: Detection of contralateral breast cancer using PET/CT (28).
When comparing enhanced CT to PET/CT according to the results of Shawky et al. (28), we can find that PET/CT has a sensitivity of 100% and a specificity of 95.4%, whereas enhanced CT has a sensitivity of 81.2% and a specificity of 90% in terms of diagnosis accuracy. Regarding distant metastases, PET/CT had a sensitivity of 100% and specificity of 80% for lymph node detection, while CT's sensitivity was 95%. In terms of identifying bone lesions, PET/CT had a sensitivity of 91.6% versus 75% for CT. For pulmonary nodules, PET/CT showed 100% sensitivity and specificity, while for hepatic deposits, sensitivity was 80% and specificity was 100%, compared to CT's 40% sensitivity.
Emerging Imaging Modalities
Artificial Intelligence (AI) is an attempt to mimic human cognitive processes using many ingredients, such as machine learning. Machine learning includes image processing algorithms for feature extraction from an input data set, and it can be categorized into two major categories: supervised and unsupervised learning (32). Supervised learning uses labeled input-output samples of the phenomenon to be modeled for classification, and classifiers (e.g., support vector machines, random forests, or neural networks. Deep learning, particularly with convolutional neural networks, is an increasingly popular trend in medical imaging, notably for the early diagnosis of breast cancer (32). Convolutional neural networks are capable of efficient feature extraction and classification due to their multi-layer composition. In unsupervised learning, however, the goal is to extract hidden structures from unlabeled data. The impact of AI on the early detection, diagnosis, and response prediction of breast cancer is tremendous and has a significant impact on patient prognosis (32). The standard performance metrics of AI in this application, including true positive, true negative, false positive, and false negative, are critical (32). AI continues to show good results being implemented in the medical field, either in predicting recurrence or identifying it. With the emerging imaging modalities, AI can help increase early detection rates and survival rates, while decreasing both mortality and morbidity rates of breast cancer.
Conclusion
Breast cancer is the third most common cancer and is responsible for a great number of deaths and disabilities worldwide. It also has a very high recurrence rate. Several imaging modalities can detect breast cancer recurrence, whether it's regional, local, or distant metastasis. Many imaging tools have high specificity and sensitivity in detecting breast cancer lesions, with the most specific tools being MRI and PET/CT. The routine screening using these tools can result in early detection, hence a better prognosis that can reach a complete resolution of tumors. Early detection of recurrent breast cancer necessitates a multidisciplinary approach, including patient education on routine screenings, annual imaging, and regular physical examinations conducted by clinicians.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding.
Ethical consideration
Non applicable.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.