Volume 2, Issue 9
September 2022
Impact of Concurrent Use of Probiotics on Risk of Antibiotic-Associated Diarrhoea in Adults
Hamed Al-Ghamdi, Maram Alaofi, Ali Al Dughman, Khalid Aldawsari, Theyab Alghamdi, Ohud Althagafi, Mohammad Alghanem, Ahmed Bin Ladnah, Kouther Almajed,Haifa Alrashed,Afia Ghazwani
DOI: http://dx.doi.org/10.52533/JOHS.2022.2902
Keywords: antibiotic, associated, diarrhoea, probiotic, concurrent
Antibiotics are one of the most prescribed medications worldwide. It is given to treat a wide range of infections and diseases. However, usage of antibiotics is associated with several side effects. The most frequently reported side effect is diarrhoea since antibiotics affect the gastrointestinal microbial environment. Antibiotic associated diarrhoea refers to the diarrhoea that develops at the initiation of the antibiotic therapy and can extend even up to six or more weeks after termination of the therapy. The prevalence of antibiotic associated diarrhoea ranges from 5% to 39% and is associated with significant morbidity and mortality. Antibiotic associated diarrhoea can be caused by any antibiotic used although the risk is higher for broad-spectrum antibiotics since they primarily target anaerobes and are poorly absorbed. Probiotics aid in reducing the risk of antibiotic associated diarrhoea. The purpose of this research is to review the available information about the impact of concurrent use of probiotics on risk of antibiotic-associated diarrhoea in adults. Various studies in the literature suggest of concurrent use of probiotics in treatment and prevention of antibiotic associated diarrhoea. Probiotics have been widely recommended as a robust and reliable strategy to lessen the negative effects of antibiotics on gastrointestinal function. Probiotics have a beneficial role in improving the gut health thus lowering the incidence of antibiotic associated diarrhoea if administered concurrently. Probiotics are generally well-tolerated and have minimum side effects. However more clinical research is needed to establish the guidelines for their concurrent use with antibiotic therapy to prevent antibiotic associated diarrhoea.
Introduction
Antibiotics are one of the most prescribed medications globally. Diarrhoea is a prevalent side effect of use of antibiotics because they affect the normal gastrointestinal microbiome. This further leads to extended hospital stays, higher rates of morbidity and mortality, and higher costs for the healthcare system (1). Antibiotic-associated diarrhoea (AAD) is defined as diarrhoea that appears between the start of antibiotic therapy and may extend to even 6 to 8 weeks after termination, can also lead to prescription noncompliance and excessive use of second-line antibiotics. Adults have an AAD prevalence that ranges from 5% to 39%. It significantly depends on the antibacterial spectrum and pharmacokinetic properties, such as the oral administration's rate of absorption and parenteral administration's enterohepatic circulation. The following two factors are part of the pathophysiology of AAD: First, antibacterial drugs have a direct impact on the intestinal mucosa. Second, antibacterial drugs interfere with the intestinal flora environment, which causes normal metabolic malfunction and an overgrowth of infections especially Clostridioides difficile (2-4).
Any antibiotic has the potential to cause AAD, although the likelihood is higher for broad-spectrum medications that primarily target anaerobes and are poorly absorbed, like clindamycin, cephalosporins, and amoxicillin-clavulanate. The prevention of AAD is one of the probiotics' most frequently prescribed purposes. In clinical research, strains from various bacterial species have been examined for AAD mitigation (5). Probiotics are live, non-pathogenic microorganisms that are administered to patients to aid with the microbial balance, especially in the digestive system. They are used as dietary supplements and foods and are made of Lactobacillus and Bifidobacterium species or Saccharomyces boulardii yeast. Reduced colonization and invasion by pathogenic organisms, reduced gut pH, and altered host immunological response are just a few of the ways that probiotics work to benefit the body (6).
Probiotics have a supportive role in the health of immune system and the digestive system. Numerous randomized controlled clinical trials have assessed the beneficial effects of probiotics on gut health in a range of disorders including antibiotic-associated and infectious diarrhea, irritable bowel syndrome, necrotizing enterocolitis, among certain others. Pathogenic microbes can be resisted by probiotics in a number of different ways. Through the mechanism of competitive exclusion, they can contend with pathogens for nutrients and adhesion sites on the gastrointestinal mucosa. By disrupting pathogen signalling by destroying quorum sensing molecules, they can also restrict or inhibit pathogenicity. The synthesis of bacteriocins or other compounds with antibacterial activity against pathogenic microorganisms can also result in direct antagonism. Probiotics can also stimulate and modify the patient's local and systemic immune responses (7).
Recent epidemiologic studies have shown a marked increase in the prevalence of AAD among healthy individuals or those who are most susceptible to the consequences, particularly older individuals. This calls for quick effort to establish a novel and practical means of prevention. Numerous systematic reviews have demonstrated that some probiotics are linked to a decreased risk of AAD, favouring the administration of probiotics along with antibiotics (8). The purpose of this research is to review the available information about the impact of concurrent use of probiotics on risk of antibiotic-associated diarrhoea in adults.
Methodology
This study is based on a comprehensive literature search conducted on August 9, 2022, in the Medline and Cochrane databases, utilizing the medical topic headings (MeSH) and a combination of all available related terms, according to the database. To prevent missing any possible research, a manual search for publications was conducted through Google Scholar, using the reference lists of the previously listed papers as a starting point. We looked for valuable information in papers that discussed the information about the impact of concurrent use of probiotics on risk of antibiotic-associated diarrhoea in adults. There were no restrictions on date, language, participant age, or type of publication.
Discussion
A typical side effect of antibiotics is diarrhoea. Adults over 65 are more prone to the development AAD. Any antibiotic may disturb the colonic microbiota and cause diarrhoea, however there is no general consensus on which antibiotics pose the most risk. Instead of happening throughout the course of treatment, AAD can happen up to 2-3 weeks after antibiotic therapy is stopped. It is a particular issue in hospitals, among older, sicker patients who are frail, and it is a very unpleasant and crippling side effect. The typical course of treatment is to stop taking antibiotics if they are still being administered. This can lead to incomplete antibiotic courses and issues treating the underlying infection, thereby lengthening the patient's stay and increasing the cost of care. Additionally, it has been demonstrated that hospital patients have a higher chance of contracting new infections in future and increased mortality (9). Different studies available in literature support the concurrent use of probiotics with the antibiotic therapy in prevention of AAD.
Literature evidence for role of probiotics on AAD
Findings of a meta-analysis in 2021 suggested that co-administration of probiotics with antibiotics lowers the incidence of AAD in adults by 37% (risk ratio =0.63 95% Confidence Interval (CI) 0.54 to 0.73, p<0.00001). Only a few species, mostly from the genera Lactobacillus and Bifidobacteria, were found to be successful in subgroup analyses, showing a favourable protective effect when high doses of the same probiotic were compared to low doses (risk ratio 0.54 (95% CI 0.38 to 0.76). Those with a low baseline AAD risk showed no difference in risk, whereas studies with a moderate or high baseline AAD risk showed a sizable risk reduction. Probiotics are successful at preventing AAD. Higher dosages and specific species have demonstrated enhanced effectiveness in secondary analyses (1). Results of another meta-analysis showed AAD was decreased in probiotic-treated groups from 16% in placebo groups to 13%. Additional subgroup analyses revealed that the protective effect was still significant when grouped by the duration, dose, and timing of the probiotics relative to the antibiotics. Probiotics demonstrated more potent protection against H. pylori treatment as compared to antibiotic therapies for other causes (10).
Blaabjerg reported in his findings of meta-analysis that use of a probiotic has been linked to a 51% reduction in the incidence of AAD (Relative Risk 0.49; 95% CI 0.36 to 0.66; I2 = 58%) and no apparent increase in the risk of adverse effects. To prevent one case of diarrhea, the number needed to treat was 11 (95% CI: 6 to 13) were needed to treat. These findings were supported by investigations of two particular strains, Lactobacillus rhamnosus GG and Saccharomyces boulardii (11). Findings of a pragmatic participatory evaluation showed that when probiotics were used, there were significantly fewer AAD episodes than when they were not administered. There were no appreciable variations in the incidence of AAD between the residents using ciprofloxacin or amoxicillin/clavulanic acid. Perceived probiotic advantages and physician prescriptions were reported as implementation aids. The use of probiotics by residents and individual selection of those who would benefit from it were reported obstacles or barrier. The effective use of probiotics in nursing home residents showed that AAD could be prevented (12).
Avadhani describes in findings of meta-analysis that in hospitalized adults, probiotics are effective in preventing AAD and Clostridium difficle associated disease. A substantial relative risk ratio was observed when probiotics and antibiotics were used together to prevent AAD and Clostridium difficle associated disease. Relative risk ratio for AAD was 44%, while for the Clostridium difficle associated disease relative risk ratio was 71% (13). Results of another study conducted by Lang reported that 199 patients in all received two sachets of the multispecies probiotic multispecies probiotic daily in addition to their antibiotic treatment after surgical surgery. By using multispecies probiotic, the prevalence of diarrhea was reduced to 0.5%. In two subjects, the probiotic administration was missed, and both developed AAD with evidence of Clostridium difficile in one subject. Hence, it was observed that the multispecies probiotic was effective for the primary prevention of AAD. By preventing AAD in this way, the entire cost of healthcare is predicted to decline (14).
Hempel stated in his study findings that probiotic use as adjunct therapy lowers the risk of AAD. Multiple subgroup and sensitivity analysis yielded consistent results. The therapeutic effect equates to 13 as number needed to treat. Interventions with Lactobacillus, either alone or in combination with other genera, make up the majority of the data base currently available for the prevention or treatment of AAD (15). Videlock revealed in his findings that probiotic medication has a preventative benefit that is consistent among probiotic species, is shown in both children and adults, and doesn't seem to depend on the other antibiotics being used concurrently or the reason an antibiotic treatment was prescribed. Even when the analysis is limited to studies with low risk of bias, the total effect is still statistically significant (16).
Probiotics are often well tolerated, with relatively little adverse effects on the digestive system. However, there have been reports of severe negative consequences such endocarditis, fungemia, and bacterial sepsis. Patients who are immunocompromised, elderly, or who have heart valve disease should utilize probiotics with caution. AAD can currently be prevented in otherwise very healthy persons taking antibiotic therapy, especially broad-spectrum antibiotics, by using probiotic products containing lactobacilli or Saccharomyces boulardii. Although side effects are often mild, elderly patients and those who have recently been hospitalized should be evaluated for the possibility of Clostridium difficle associated disease so that self-care can be substituted for a referral to a primary care physician (17).
Milner stated that probiotics have been widely recommended as a robust and reliable strategy to lessen the negative effects of antibiotics on gastrointestinal function, making them a popular alternative for people with AAD. One of the main causes of AAD is Clostridium difficle associated disease, which can develop after the loss of intestinal flora brought on by antibiotic use and perhaps worsen acute diarrheal sickness. The potential of probiotic use for preventive or therapeutic applications in a variety of gastrointestinal diseases is highlighted by the metabolic importance of the gut microbiota to food and drug pharmacokinetics. Probiotics have been demonstrated to improve the intestinal epithelial barrier and lessen inflammation from a molecular standpoint. Although some probiotics undertaking clinical trials to treat complicated gastrointestinal and inflammatory illnesses have shown promising outcomes, the conventional drug development paradigm linked to preclinical and clinical investigations is lacking (18).
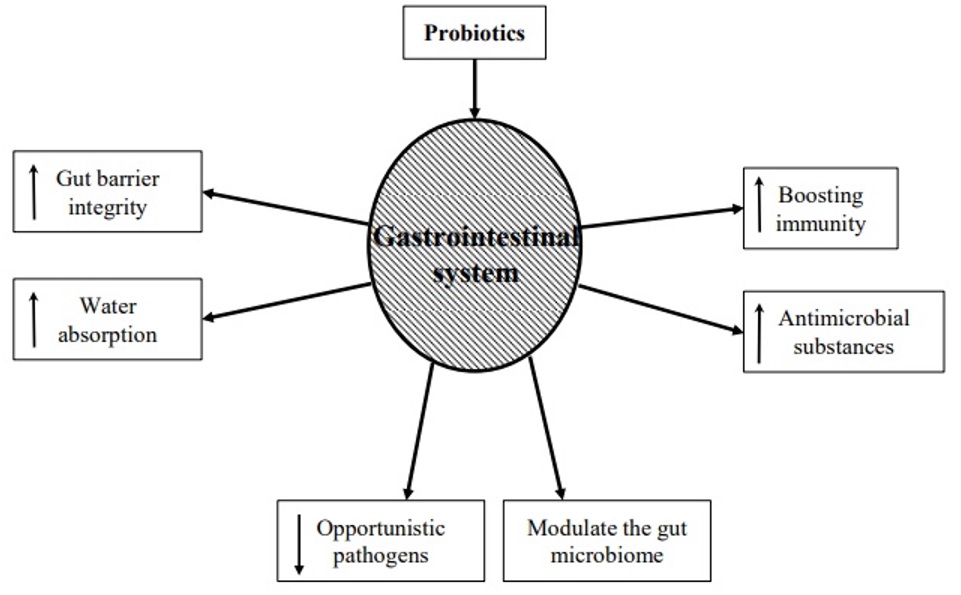
Numerous research has demonstrated the advantages of using probiotics for AAD. It has been demonstrated that using probiotics can restore gut microbiome dysbiosis. When a patient is subjected to extreme circumstances like protracted antibiotic medication, excruciating physical stress, and persistent sickness, dysbiosis may develop. Complex carbohydrates are broken down by probiotics into lactic acid and short-chain fatty acids. This lessens bacterial translocation, enhances the integrity of tight junctions, and promotes the formation of mucin (19). Probiotics acts through various mechanisms to treat AAD. Hypothesized role of probiotics in treatment of AAD is illustrated in (Figure 1). The findings of numerous meta-analyses and systematic reviews have fuelled the expanding use of probiotics. These studies' findings imply that using probiotics together with antibiotic therapy lowers the incidence of AAD and Clostridium difficle infection. Probiotics should only be given to patients who are most at risk of developing Clostridium difficle infection and AAD, according to the available data including patients receiving multiple antibiotics for long durations. Additionally, it appears that the most evidence supports the usage of Lactobacillus rhamnosus GG. When antibiotic therapy begins, these medications should be started, and they can be stopped when treatment is over. The possible hardship and expense that probiotics may provide to patients should be carefully considered by healthcare professionals because these aspects may affect antibiotic adherence. Immunocompromised patients, in whom probiotics have been linked to illnesses such bacteremia, should avoid using them (20).
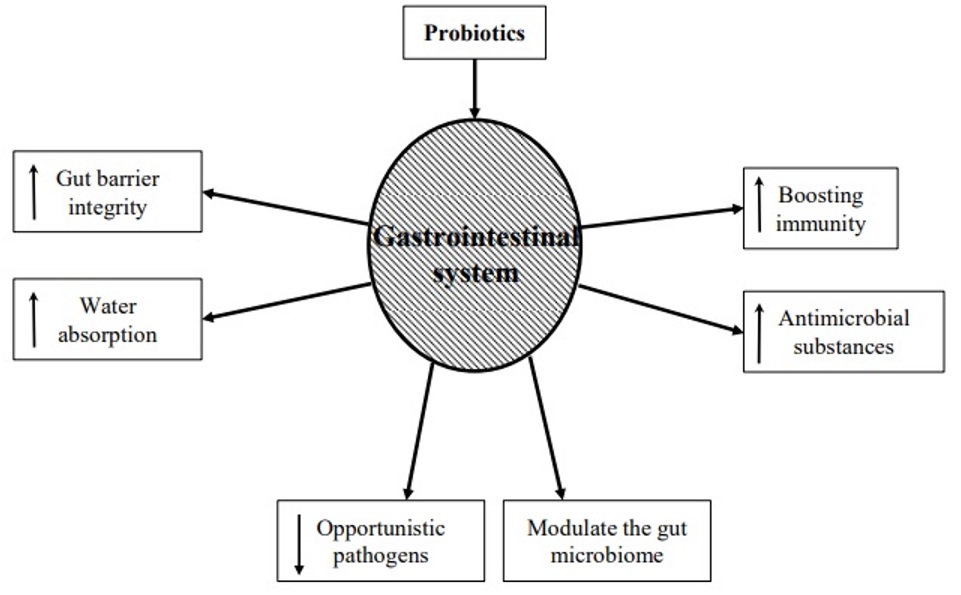
Figure 1: Hypothesized role of probiotics in the treatment of AAD (19).
Issa stated that probiotics have a potent demand due to their easy accessibility, low cost, and well-known safety profile. However, with the available information, it is challenging to reach any firm conclusions regarding the prophylactic use of probiotics in AAD as more research is required. In some specific populations, such as those with a history of AAD or risk factors for the emergence, it may be prudent to advise their usage. Many physicians have been reluctant to incorporate probiotics into their regular practices; at this time, it would be appropriate to stratify this use on a case-by-case basis.(21) Literature recommends the role of probiotics in prevention of AAD however majority of the studies available are systematic reviews and meta-analysis, in order to generate evidence-based guidelines for the concurrent use of probiotics for the prevention of AAD more clinical research is needed including large or population based placebo-controlled trials.
Conclusion
Probiotics may be taken concurrently with the course of antibiotic therapy to prevent antibiotic associated diarrhoea in adults. Although, for establishment of precise recommendations and guidelines, future research should pay attention to the strain specificity and concentrate on the ideal probiotic dosage and duration.
Disclosure
Statement
The authors declare no conflict of interest.
Funding
No funding.
Ethical consideration
Non-applicable.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Authors’ contribution
All authors contributed equally to the drafting, writing, sourcing, article screening and final proofreading of the manuscript.