Volume 2, Issue 8
August 2022
Urinary Tract Infections Among Geriatric Patients: A Systematic Review
Husam Alamri, Suliman Alnamlah, Waleed Altulayqi, Eyad Owaydhah, Abdullah Almohammadi, Ammar Allam, Awnallah Alotaibi, Raghad Alsayed, Abdulrahman Albejawi
DOI: http://dx.doi.org/10.52533/JOHS.2022.2806
Keywords: Elderly Patients, Urinary Tract Infection, Geriatric, Systematic Review, Prophylaxis
Urinary tract infection (UTI) is the second most popular infection in the geriatric community, and the first popular is urine and fecal incontinence. Because of the effects of immunological aging, elderly are more susceptible to bacterial microorganisms and viral infections. The disease ranges from relatively harmless cystitis to potentially fatal pyelonephritis. This article aims to discuss the epidemiology, diagnosis, risk factors, progress of the disease, causative microorganisms, and guidelines for drug management of UTIs in geriatric patients. This study was performed following the PRISMA checklist. PubMed (2016-2022), Biological Sciences (ProQuest) (2012-2022), and ScienceDirect were used to locate English-language literature (2018-2022). We extracted data to assess the epidemiology, diagnosis, risk factors, causative microorganisms, and treatment management of UTIs in geriatric patients. This systematic review encompassed 11 observational studies including a total of 945,908 elderly patients and were published between 2012 and 2022. The prevalence varies across the studies. Urinary catheterization, gender, polypharmacy, comorbidities especially diabetes mellites older age, dementia, older age, vaginitis, previous history of UTI, bedridden state, and antimicrobial use were the independent risk factors. E. coli was the most found causative agent. Antibiotics were given specifically for UTIs. Despite the high rate of risk factors and prevalence of UTIs in the geriatric, treatment options are minimal. There is no evidence to support the use of antibiotics for long-term UTI prevention. However, alternative prophylaxis methods for patients with recurring infections must be developed. Although further studies are required to properly understand the clinical features and treatment modalities in elderly patients within the community or under institutional care.
Introduction
Urinary tract infections are highly prevalent, affecting nearly 150 million people worldwide annually. However, urinary tract infections are commonly present and the second most popular infection in the geriatric population, due mainly to their greater susceptibility to microbial infections and viral infections (1). They are also more susceptible to infections due to dehydration, incontinence, limited activity, and impaired cognitive function (2). Bacterial growth in urine without signs of the urinary tract is the most frequent form of the symbiotic colony (asymptomatic bacteriuria). In healthy women before menopause, 1-5% of asymptomatic bacteriuria is present, in 4-19 % of healthy elderly women, and in men about 15-50 % of hospitalized elderly (3).
Diagnosis and treatment of UTIs in older patients is more difficult than in younger patients associated with major variables such as age, metabolic disorders, diabetes Mellitus (DM), nerve damage, the catheter of the urethra, and general health. Unusual symptoms can also contribute to a late diagnosis. Rectocele, cystocele, and pelvic prolapse are all possible complications (4). Urinary tract infections are caused by ureteral reflux, bladder diverticulum, poor perineal hygiene, incontinence, vaginal atrophy, estrogen deficiency in women, and prostate diseases in men. Changes in mental status, diabetes, immunosuppression, invasive procedures, neurological diseases, strictures, and anatomical changes are all major risk factors in the elderly (5, 6).
Urinary tract infections, dysuria, pollakiuria, and urgency are all common in geriatrics. However, nausea, incontinence, pain in the abdomen, vomiting, distress in the respiratory system, and changes in consciousness may lead to a missed diagnosis (1). The typical symptoms of UTI, as well as the difficulty in treating infections once diagnosed, can lead to increased hospitalization and mortality in the elderly. As a result, in the diagnosis of UTI in the elderly, physician suspicion is important (7). As a result, geriatric patients are subjected to a pre-treatment urine culture and a urinal dipstick test when clinical suspicion exists. Both the use of a urinal dipstick test to determine predictive values and empirical antibiotic treatment have been shown to reduce morbidity and mortality (8).
This systematic research investigates the science-based clinical evidence, diagnostic strategies, complicating factors, progression, and pathogenic microorganisms. The goal was to highlight a few main points about urinary infections in the geriatric and have a discussion about their suitable management.
Methodology
Definition of outcome and inclusion criteria
The clinical evidence, diagnostic approaches, complicating factors, pathogenic microorganisms, and therapeutic options for UTIs in geriatric patients were the focus of our investigation. We included observational studies either cohort or cross-sectional designs; assessing the prevalence, diagnostic strategies of symptoms and /or signs in predicting UTI; providing a reference standard for confirming the diagnosis of infection; causative agent, and treatment management in patients over 65 years. Also, we included investigations in which a small proportion of participants were aged under 65 (>60) years. We excluded studies in which not published in English; with non-human subjects; case reports and case series
Search strategy
Based on our determined outcomes, we retrieved the relevant keywords from a brief manual screening within the potentially included studies to design the most suitable search term. We used keywords such as urinary tract infections, acute uncomplicated cystitis, asymptomatic bacteriuria, catheter-associated urinary tract infections, acute uncomplicated pyelonephritis, antibiotic resistance, and recurrent urinary tract infections in the elderly. We performed searches in PubMed (2016-2022), Biological Sciences (ProQuest) (2012-2022), and ScienceDirect (2018-2022). No restrictions were applied for country, ethnicity, or sex. Language restrictions were applied. Our search method was restricted to the search results' titles and abstracts to only use pertinent studies. To find all duplicates across the various searched databases, all these results were exported to an Endnote library. The ensuing articles' citations led to the discovery of other publications. This systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol at all stages. (9).
Screening and extraction
We performed a double screening strategy—one for screening titles and abstracts and the other for screening full texts to maintain high quality in this important process. After ensuring that all relevant articles were included, an extraction sheet was constructed in an organized way relevant to our aimed outcomes. The sheet was composed of the baseline characteristics and the sought variables.
Quality Assessment
Other information that was extracted from the included studies also included data about the potential risk of bias in these studies. We used the modified Newcastle-Ottawa scale (NOS) for observational studies (10), which primarily consist of following parts, including the quality of methods, assessment, compatibility, and reporting of the results. Studies were rated as outstanding, good, satisfactory, or not satisfactory on a scale of 0 to 10, depending on how biased they were.
Results
Search Results
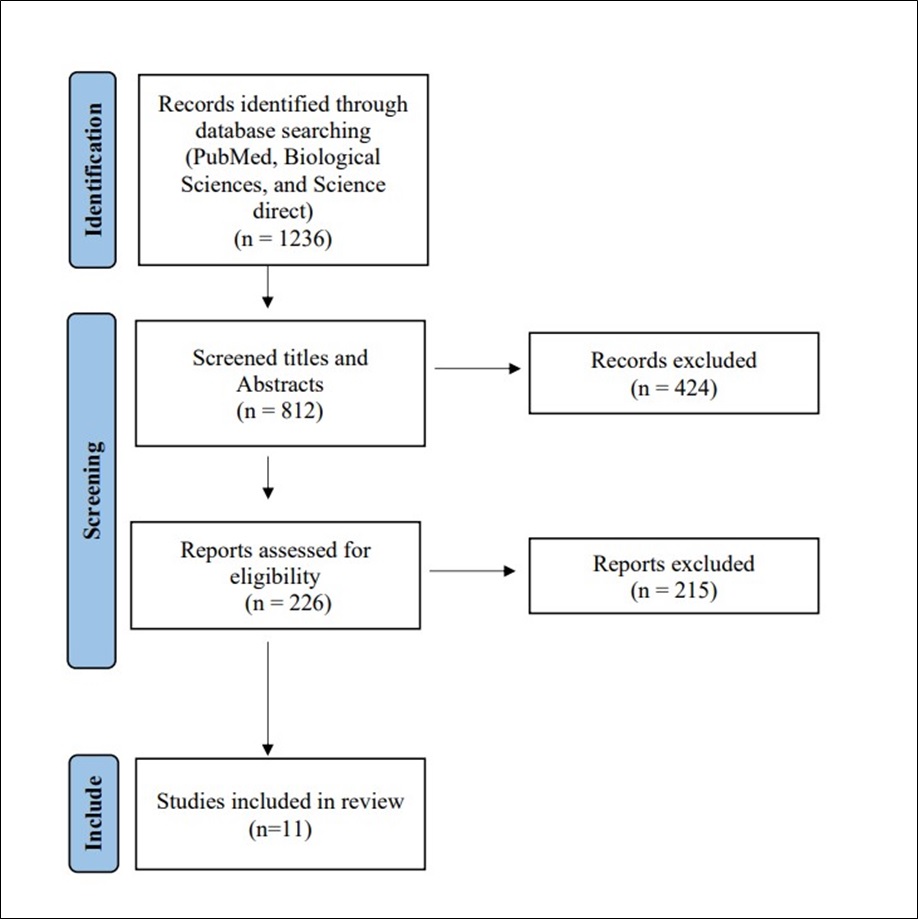
We were able to uncover a total of 1236 citations using the aforementioned search techniques, which were subsequently reduced to 812 after duplicates were eliminated. Only 226 citations remained after the title and abstract screening that qualified for the following stages. Only 11 articles met our inclusion and exclusion criteria after the full-text screening. The comprehensive search plan and screening are displayed in Figure 1.
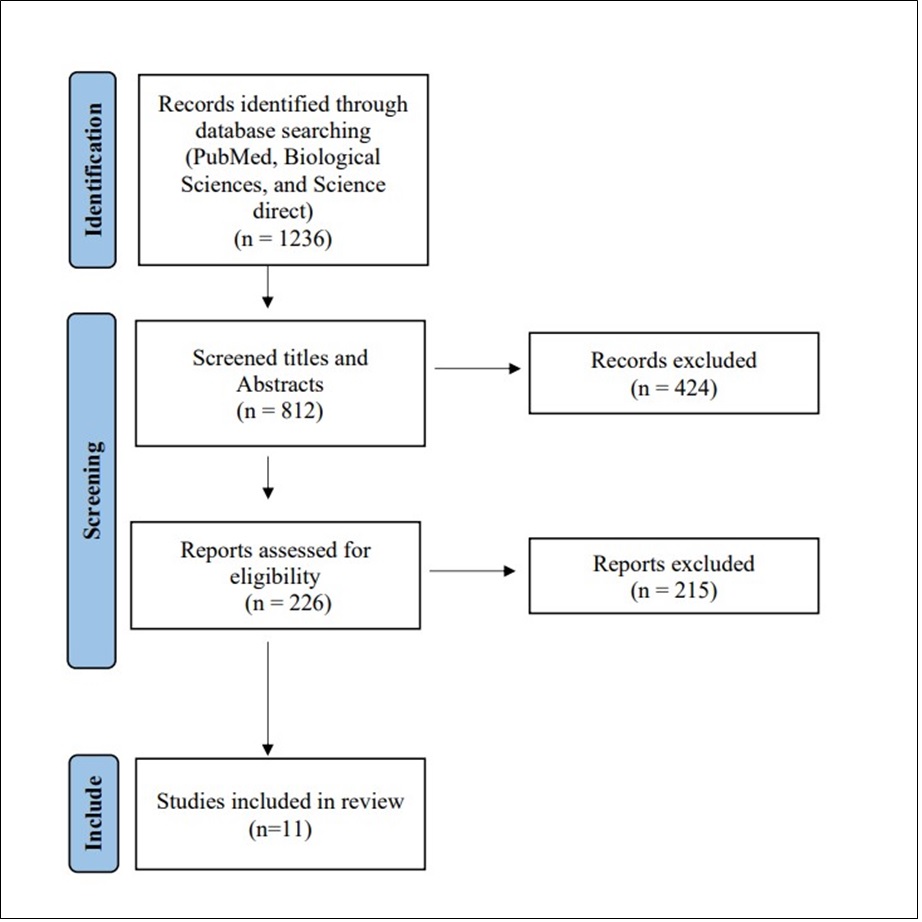
Figure 1.PRISMA flow chart
Results of quality assessment
According to our assessment of bias for the included investigations, the majority of them had satisfactory quality and a low risk of bias, while the remaining ones had good quality. The included studies showed neither excellent nor unsatisfactory results. The detailed results of the quality assessment according to the adjusted NOS tool are shown in Table 1.
|
Table 1: Summary of the results of bias assessment of the included studies using the modified Newcastle-Ottawa scale (NOS) for observational studies. |
||||||
|
No |
Author |
Selection of the study group |
Comparability |
Ascertainment of the exposure and outcome |
Total Score |
Study Quality |
|
1 |
Gajdács et al |
*** |
** |
5 |
fair |
|
|
2 |
Aktar et al |
*** |
* |
** |
6 |
good |
|
3 |
Kbirou et al |
*** |
** |
5 |
fair |
|
|
4 |
Manseck et al |
*** |
* |
** |
6 |
good |
|
5 |
Ioannou et al |
*** |
* |
** |
6 |
good |
|
6 |
Petty et al |
*** |
** |
5 |
fair |
|
|
7 |
Bagaria et al |
*** |
* |
** |
6 |
good |
|
8 |
Swami et al |
*** |
** |
5 |
fair |
|
|
9 |
Ahmed et al |
*** |
* |
** |
6 |
good |
|
10 |
Alpay et al |
*** |
** |
5 |
fair |
|
|
11 |
Marquez et al |
*** |
** |
5 |
fair |
|
Characteristics of the included studies
We included 11 trials that recruited 945908 patients and were published between 2012 and 2022. All the studies that were considered were observational, and 81% of them were retrospective. The United Kingdom, Germany, Morocco, Turkey, Malaysia, the United States, Hungary, Brazil, and Greece were all represented in each study, whereas India was the country for two of the included studies. Table 2 displays all of these studies’ baseline characteristics.
|
Table 2: Baseline characteristics of the included studies in this review. |
||||||||
|
No |
Author |
Year |
Country |
Study Design |
Study Type |
Sample size |
Age |
Gender (Male/Female) |
|
1 |
Gajdács et al |
2021 |
Hungary |
Observational |
Retrospective |
9166 |
>65 |
NR |
|
2 |
Aktar et al |
2021 |
Malaysia |
Observational |
Retrospective |
460 |
>65 |
39.35%/60.65% |
|
3 |
Kbirou et al |
2021 |
Morocco |
Observational |
Retrospective |
321 |
Average: 61 |
NR |
|
4 |
Manseck et al |
2020 |
Germany |
Observational |
Retrospective |
126 |
>75 |
NR |
|
5 |
Ioannou et al |
2020 |
Greece |
Observational |
Retrospective |
204 |
Average:83.3 |
38.73%/61.27% |
|
6 |
Petty et al |
2019 |
United States |
Observational |
Retrospective |
2733 |
Median: 77 |
78.2%/21.8% |
|
7 |
Bagaria et al |
2019 |
India |
Observational |
Prospective |
95 |
>60 |
55.78%/44.22% |
|
8 |
Swami et al |
2018 |
India |
Observational |
Retrospective |
120 |
>60 |
58.2%/41.8% |
|
9 |
Ahmed et al |
2018 |
United Kingdom |
Observational |
Retrospective |
931945 |
>65 |
44.77%/55.23% |
|
10 |
Alpay et al |
2018 |
Turkey |
Observational |
Retrospective |
140 |
>65 |
46.4%/53.6% |
|
11 |
Marquez et al |
2012 |
Brazil |
Observational |
Prospective |
598 |
Average:71.93 |
all female |
NR: not reported
The prevalence varied across the studies. One study evaluated person-year risk and incidence trends over the years. The commonly observed causative agents were gram-negative organisms, especially E. coli. Older age, catheterization, comorbidities, dementia, acutely altered mental status, leukocytosis, and the presence of leukocyte esterase were the associated risk factors. Polypharmacy and the presence of comorbidities were identified as potential risk factors for UTI treatment outcomes. Gender, bedridden status, dementia complicated UTI, diabetes, and previous hospitalization were found to be independent predictors of mortality in elderly UTI patients. The details of the causative agents and the treatment management are displayed in Table 3.
|
Table 3: Summary of the outcomes of the included studies in this review. |
|||||
|
Author |
Clinical Features/Etiology |
Incidence/ prevalence |
Risk factors |
Causative agent |
Management |
|
Gajdács et al |
NR |
NR |
NR |
Outpatient: Enterococcus spp. (20.15%), E. coli (48.14%), Klebsiella spp. (16.28%), Proteus-Providencia-Morganella group (4.56%), Pseudomonas spp. (4.40%); Inpatient: Enterococcus spp. (21.52%), E. coli (25.65%), Klebsiella spp. (16.26%), Proteus-Providencia-Morganella group (10.96%), Pseudomonas spp. (13.36%) |
higher resistance rates were observed in inpatient isolates for many Access and Watch antibiotics compared to isolates of outpatient origin |
|
Aktar et al |
NR |
cystitis (37.6%) ASB (31.9%), pyelonephritis (13.9%), urosepsis (10.2%), and prostatitis (6.4%) |
Gender (female), polypharmacy, comorbidities |
NR |
Ampicillin and Sulbactam (57.1%), Trimethoprim/sulfamethoxazole (31%), Ciprofloxacin (5.4%), Levofloxacin (3.7%), Cloxacillin (2.6%), Hytrin (Terazosin) (84.8%) |
|
Kbirou et al |
Bladder tumour (37%), Adenoma of the prostate (32%), Prostate tumour (19%), Stenosis of the urethra (8%), Neurological bladder (4%) |
66% |
urinary catheterization (67%) |
E. coli (54%), K. pneumoniae (17%), Pseudomonas aeruginosa (9%), Proteus mirabilis (6%), Enterobacter cloacae (3%), Enterococcus faecalis (5%), staph aureus (4%), Enterococcus faecium (2%) |
low sensitivity to Ampicillin (23%), Amoxicillin + Clavulanic acid (31%), a moderate sensitivity to Quinolones (68%), Nitrofurans (62%), and Trimethoprim (61%), and high sensitivity to aminosides (78%) (amikacin and gentamicin), imipenem (96%), and Colistine (98%) |
|
Manseck et al |
NR |
14% |
NR |
E. coli (49.7%), Klebsiella spp. (10.3%), Proteus spp. (9%), Pseudomonas aeruginosa (7.6%), Staphylococcus spp. (6.2%), Enterobacter spp. (4.1%), Citrobacter spp. (2.8%). |
Ciprofloxacin (34.9%), Amoxicillin-clavulanic acid (24.6%), Fosfomycin (12.7%) Cefuroxime (8.7%), Amoxicillin (8.7%), Trimethoprim (4.8%) |
|
Ioannou et al |
NR |
NR |
antimicrobial use, hospitalization, dementia, bedridden state, and sex |
E. Coli (40.5%), Klebsiella pneumoniae (13.7%), Proteus mirabilis (11.7%), Enterococcus faecalis (10.7%), Pseudomonas aeruginosa (5.4%), Enterococcus faecium (2.4%), Candida albicans (2.4%), Acinetobacter baumannii (1.5%), Morganella morgannii (1.5%) |
Piperacillin/tazobactam (42.2%), Quinolone (36.3%), Cephalosporin (26.5%), Carbapenem (16.7%), Aminoglycoside (9.3%), Vancomycin (8.3%) |
|
Petty et al |
Abdominal pain (6.7%), abnormal urine analysis (42.6%), fatigue (6.9%) |
NR |
Older age, dementia, acutely altered mental status, leucocytosis, presence of leukocyte esterase |
E. coli (50.2%), Klebsiella spp. (15.3%), Enterococcus spp. (10.8%), Proteus spp. (5.6%), Pseudomonas aeruginosa (3.8%), Enterobacter spp. (3.5%), p 97 (3.5) |
Ceftriaxone (61.6%), Fluoroquinolone (18.9%), Cephalexin (3.2%) At discharge: Fluoroquinolone (33.2%), Cephalosporin (1st or 2nd generation) (31.5%), Trimethoprim with sulfamethoxazole (9.4%), Nitrofurantoin (6.9%) |
|
Bagaria et al |
fever with chills urgency, dysuria, pyuria, haematuria, |
NR |
diabetes mellitus, complicated UTI |
Gram-negative organisms (92.63%), gram-positive (7.36%), E. coli (47.36%), Klebsiella group (18.94%) Pseudomonas (14.73%) |
Imipenem |
|
Swami et al |
Dysuria was the most common major symptom (77.5%) |
NR |
older age, diabetes mellitus |
Gram-negative organisms were responsible for (68.27%) of the uropathogen profile; E. coli (31.66%) |
NR |
|
Ahmed et al |
NR |
21% |
person year risk was calculated |
NR |
Trimethoprim, Tetracyclines, cephalosporins, Penicillin, Quinolone, nitrofurantoin, Macrolides |
|
Alpay et al |
Fever (77%), Nausea (29%), Vomiting (15%), Abdominal pain (14%), Polyuria (29%), Dysuria (56%), Pyuria (94%), Haematuria (77%), Secondary bacteremia (9%), Antibiotic exchange rate (15) |
25.09% |
NR |
Escherichia coli (66%), Klebsiella pneumoniae (15%), Pseudomonas aeruginosa (8%), Enterococcus spp. (6%), Acinetobacter baumannii (3%), Staphylococcus aureus (1%), Candida spp. (1%), Contamination/no growth (43.6%) |
Amoxicillin/clavulanic acid, Cefepime, Ceftazidime, Cefotaxime, Ceftriaxone, Amikacin, Piperacillin/tazobactam, Colistin, Cefoperazone/sulbactam, Tobramycin, Imipenem, Ciprofloxacin, Gentamicin, Trimethoprim/sulfamethoxazole |
|
Marquez et al |
Foul smelling urine (60.6%), Dysuria (33.33%), Frequency (30.30%), Urgency (29.29%) |
16.50% |
age, history of previous UTI, vaginitis, diabetes |
E. coli (75.75%), Enterococcus spp. (9.09%), Proteus mirabilis (6.06%), Klebsiella pneumonia (5.05%), Staphylococcus saprophyticus (3.03%), Citrobacter freundii (1.01%) |
NR |
NR: not reported, UTI: urinary tract infection, ASB: asymptomatic bacteriuria
Discussion
Prevalence and incidence
In community-dwelling geriatric women, UTIs are the most common type of infection, as well as in hospitalized or long-term care geriatric women. Furthermore, women have a higher rate of UTIs than men, thus possibly due to a shorter urethra that allows bacteria from the intestine to pass through more easily (11).
In the community, there are 7 UTI cases per 100 geriatric women every year. These results are according to a prospective observational study of postmenopausal women (ages 55-75). A study of asymptomatic bacteriuria in 46 centers in the US discovered that 78.2% were female patients (12). The risk factor of UTI in females was 1.7-fold higher than in males (incidence per 100 person-years was 12.8) in a Dutch study of people over the age of 85 (13). Catheter-associated UTIs were found to be higher in patients aged over 60 years (66%) (14). Cystitis was the most frequently reported UTI type in elderly patients, followed by asymptomatic bacteriuria, pyelonephritis, urosepsis, and prostatitis (15).
The occurrence of UTIs rises with age. The incidence increased from 9–11 cases per 100 person-years to 11.4–14.3 cases in women aged 65–74 years. Men's values ranged from 2.8–3.0, 5.9–6.7, and 8.1–10.5 (11). Over the age of 85, both genders have a marked increase in UTI prevalence.
Populations in the community
The classic signs and symptoms of a urinary tract infection are frequently seen in elderly people who live in the community. Lower tract irritative symptoms such as frequency, urgency, and dysuria characterize cystitis. Abdominal pain or tenderness in the costovertebral angle distinguishes pyelonephritis (16). Fever with chills, backache, nausea, vomiting, polyuria, pyuria, and fatigue were the other symptoms found in the included studies of the current review.
Institutionalized people
In elderly patients, bacteriuria is most often misdiagnosed as symptomatic urinary tract infections. Residents of long-term care facilities can also exhibit these classic symptoms. A urinary tract infection is unlikely in a bacteriuric resident who does not have localized genitourinary symptoms (17). Increased incontinence or dehydration are frequently misdiagnosed as UTIs. Bacteriuria is associated with changes in the appearance of urine, such as odor, color, or turbidity. These changes aren't enough to rule out asymptomatic UTIs. In one included study, 60.6% of patients reported foul-smelling urine (18).
Before starting antimicrobial therapy for a urinary infection in a resident who does not have an indwelling catheter, acute localized genitourinary signs or symptoms must be present. Acute dysuria is a distinct clinical manifestation that can occur alone or in conjunction with fever (19-21), a genitourinary symptom that is new or worsening, as well as acute confusion or chills. The procedure was found to be safe for patients and resulted in less antimicrobial use for urinary infections (22). A significant diagnostic challenge is an elderly patient who presents with acute confusion and no localized findings. Because the resident does not have a chronic catheter, urinary infection is unlikely. It may be preferable to treat the condition as a "sepsis syndrome" with no known cause (23).
Indwelling catheter (chronic)
Fever is the most typical symptom of a UTI in patients with an indwelling catheter. There may be costovertebral angle pain or tenderness to support the genitourinary source. Catheter obstruction or hematuria may also occur (24). In the absence of another source, the consensus definitions for clinical presentations to initiate empiric antimicrobial therapy for residents with chronic catheters only recommend the presence of one of the following symptoms: Fever, new costovertebral angle tenderness, rigors, or new-onset delirium are all possible symptoms (25, 26).
Guidelines state that the chance of a catheter-associated UTI should not be predicted by symptoms or indications alone. Because the majority of catheterized patients have bacteriuria, only send catheter urine samples if the patient exhibits signs of sepsis. Before beginning antimicrobials, urinary catheters should be removed and a culture obtained from a newly inserted catheter (14).
In elderly patients in long-term care who have catheters, antibiotics should be started as soon as possible. Among the criteria were fever > 37.9°C or 1.5°C above baseline temperature, new costovertebral tenderness, rigors without an obvious cause, or new onset of delirium. According to Scottish Intercollegiate Guidelines Network (SIGN) guidelines, clinicians should look for signs of infection in pyrexial patients with urinary catheters. To isolate the infectious organism and test antibiotic sensitivity, a urine sample should be cultured. The severity of the infection, as well as any underlying illnesses, must be considered (27).
When a catheter has already been in place for longer than a week, the Infectious Diseases Society of America (IDSA) advises changing it before beginning antibiotic treatment in catheterized patients who are exhibiting symptoms. According to IDSA recommendations, catheters that have been in position for more than two weeks should be removed to hasten symptom relief and avoid UTI recurrence. The most effective ways to reduce the risk of catheter associated UTIs are to reduce the use of indwelling catheters and to remove catheters when they are no longer clinically necessary (28).
Risk factors for UTI bacteriuria
Symptomatic UTIs are more common in geriatric people than in the younger population. Age and exposure to nosocomial pathogens with variations in immune function are the main two risk factors for developing urinary tract infection. There is no doubt that the risk of urinary tract infection remains extremely high in all age groups. Asymptomatic bacteriuria and UTIs in hospitalized geriatric populations are more common. Hospitalized elderly patients have more comorbidities and functional impairments than other communities. In addition, hospitalized elderly people are at a significant risk factor for UTI if they have a urinary catheter (29). Kbiroul et al., (14) investigated the relationship between urinary catheterization and catheter-associated urinary tract infections. They concluded that to ensure adherence to best standards and hence lower the risk of urinary tract infection, strict urine catheterization procedures are required.
Geriatric patients (aged 65 or over) are more likely to acquire UTIs due to a number of intrinsic and extrinsic risk factors, and they frequently put off getting treatment. Intrinsic risk factors include age-associated immune senescence and existing illnesses that impair immunity (such as diabetes or cancer), urinary incontinence, benign prostate hyperplasia, malnutrition, and immobility. Hospitalization, catheterization of the urinary tract, and chemotherapy are extrinsic risk factors. According to Akhtar et al., (15) female patients, polypharmacy, and the presence of comorbidities were the potential risk factors for the treatment outcomes of UTIs. However, a multivariate logistic regression analysis model identified male sex, bedridden state, dementia complicated UTI, diabetes, and previous hospitalization as independent factors associated with mortality in elderly UTI patients (20, 30). In Table 3, other risk factors for UTIs in older adults are listed. Indeed, persistent urinary tract infections (UTIs) in women have been linked to estrogen deficiency after menopause. Recurrent UTI is linked to cystoceles, large postvoiding residual urine volumes, and incontinence. Urinary symptoms and urinary retention are symptoms of prostate disease in men. Urinary infection risk is increased by any urological disorder that can result in blockages, such as stones or tumors (14).
Microbiology
Urinary tract abnormalities and complications are more common in the geriatric population. There are two types of UTIs: complicated and uncomplicated (31). A complicated UTI is distinguished by structural or functional abnormality of the urinary tract. The most frequent cause of urinary tract infections is Gram-negative organisms in the perineal region that are ascending through the urethra from bowel flora (11, 12, 20, 32). In most of the included studies has been found the higher percentage of E. coli in urine culture ranges between 40% and 75.75%. The enterobacterales family, specifically E. coli, Proteus mirabilis, and Klebsiella pneumonia, is responsible for the majority of microbial isolates found in geriatric patients with UTIs (12, 14, 18, 21, 22, 30).
According to evidence, these organisms are involved in more than 90% of cases of cystitis in older adults (33). E. coli is the predominant organism isolated from symptomatic infection in elderly men and women. The other bacteria are Enterococcus spp. and Klebsiella pneumoniae isolates in elderly UTIs. E. coli virulence determinants isolated from patients with symptomatic cystitis or pyelonephritis are similar to those described for strains isolated from younger women (34). Enterobacteriaceae such as K pneumoniae, Citrobacter spp., Serratia spp., Enterobacter spp., and non-fermenters such as Pseudomonas aeruginosa are among the other species (12).
In patients, the Proteus-Providencia-Morganella group (10.96%) and Pseudomonas spp. (13.36%) were isolated more in in-patients than outpatient UTIs. While E. coli (48.14%) has been isolated more in outpatient UTIs (35). Candida spp. has been noticed in diabetic patients with indwelling urologic devices, and those taking broad-spectrum antibiotics on a rare basis. E. coli is still the typical cause of asymptomatic or symptomatic UTIs in women in long-term care homes. Men have been isolated with (36) Proteus mirabilis, Providencia stuartii, coagulase-negative staphylococci, and Enterococcus spp.
Management of UTI
While UTIs are common among the elderly, there are limited recommendations for diagnosis, treatment, and management. The empirical management of UTIs in older individuals has been outlined in antibiotic (therapeutic) guidelines. A history of any antibiotic allergies should be reviewed before starting antibiotics. Depending on the patient's renal function, antibiotic doses may need to be adjusted (20).
There is a higher incidence of multi-drug resistant bacteria in hospitals due to increased antibiotic prescribing and repeated exposures to healthcare-acquired pathogens during hospitalization. Patients over the age of 65 with a UTI should receive antimicrobial protection as part of their empirical treatment (37). In this review, one included studies that reported the duration of treatment, such as Nitrofurantoin used as oral therapy for seven days to treat cystitis, and an intravenous amoxicillin-clavulanic acid followed by oral therapy was used for 14 days to treat pyelonephritis (18). In another included study in this review, they analyzed the patterns of empiric antibiotic prescriptions. Apparently, over the course of the study, fewer broad-spectrum antibiotics were prescribed. The percentage of elderly men (from 45% to 74%) and women (from 55% to 82%) who had UTIs and were given antibiotics specifically for UTIs increased (11). Table 4 summarizes the empirical treatment recommendations for women with community-onset UTIs based on the WHO, ESCEO, and NICE guidelines. Patients who have had a lot of medical contacts or who live in nursing homes, on the other hand, might get different treatments (38, 39). Clinical criteria for initiating empiric antimicrobial therapy were recommended in a consensus guideline (40).
|
Table 4: Empirical treatment recommendations for community-onset UTI in women |
|
|
Antibiotic |
Dosage and course length |
|
First choice |
|
|
Nitrofurantoin |
every 6 h for 3 days,100 mg orally. |
|
Cefalexin |
every 12 h for 5 days, 500 mg orally, |
|
Trimethoprim |
daily for 3 days, 300 mg orally |
|
Second-best (no improvement in lower UTI symptoms on first-choice antibiotic taken for at least 48 h, or when first-choice not suitable) |
|
|
Amoxicillin |
500 mg orally, every 8 h for 5 days |
|
Amoxicillin + clavulanic acid |
500 + 125 mg orally, every 12 h for 5 days |
|
Trimethoprim + sulfamethoxazole |
160 + 800 mg orally, every 12 h for 3 days |
Due to a lack of evidence to guide antibiotic therapy, men's urinary tract infections are uncommon. UTIs are more common in older men and are frequently associated with urinary tract abnormalities. UTIs are treatable with a variety of antibiotics, but resistance to ampicillin, cephalexin, and amoxicillin-clavulanate is on the rise (4). When patients are hospitalized, this resistance necessitates the use of broad-spectrum antibiotics. Antibiotic susceptibility data should also be used to assist in the selection of antibiotic agents. Allergy history, side-effect profile, drug interactions, physiological factors such as impaired renal function, and administration frequency (daily vs multiple daily dosing) are all important considerations (41).
Management of recurrent UTI
Patients over 65 were associated with the recurrent sequel of UTIs and longer hospitalization (35). To reduce the frequency and severity of recurring UTIs, prophylactic antibiotics may be prescribed. However, the long-term consequences, such as side effects, recurrence rates, and antimicrobial resistance, are still unknown. According to a Cochrane review (42) prophylaxis reduced clinical and microbiological recurrences. According to evidence from several small trials with relatively short follow-up periods, antimicrobial therapy appears to reduce the risk of UTI recurrence in postmenopausal women. Recurrent urinary tract infection is distinct from recurrent asymptomatic bacteriuria and should be further investigated, particularly in men (43).
After one month of prophylactic treatment, more than 90% of urinary and fecal E. coli isolates developed resistance to trimethoprim-sulfamethoxazole. There is no published evidence to support the use of antimicrobial 'cycling' with long-term continuous prophylaxis at the moment. For up to 6 months, therapeutic guidelines recommend either intermittent or continuous post-coital prophylaxis with a single agent (44).
The European Medicines Agency recommends that high-strength estradiol vaginal creams be used for a single four-week treatment period. Higher-than-normal levels can cause side effects similar to those seen with systemic hormone replacement therapy after four weeks. Intravaginal estrogen replacement and prophylactic antibiotic therapy can both be utilized to prevent recurrent UTIs (18). Intra-vaginal estrogen therapy can help restore normal vaginal flora and reduce the risk of E. coli colonization (45). In patients with urinary tract infections, intravenous administration of hyaluronic acid or a non-pathogenic strain of E. coli is beneficial. These alternative approaches could be discussed with patients who have recurring infections that are resistant to treatment (46, 47).
Conclusion
Despite the high rate of risk factors and prevalence of UTIs in the geriatric, treatment options are minimal. In addition to being more prone to UTIs, elderly persons are also more likely to have concomitant conditions and need multiple drugs. The effects of the disease, patient age, comorbidities, existing external devices, local patterns of antibiotic resistance, and patient compliance with the therapy must all be considered when selecting a treatment plan. However, there is no evidence to support the use of antibiotics for long-term UTI prevention currently. However, alternative prophylaxis methods for patients with recurring infections must be developed. Although further studies are required to properly understand the clinical features and treatment modalities in elderly patients within the community or under institutional care.
Disclosure
Statement
The authors declare no conflict of interest.
Funding
No funding.
Ethical consideration
Non-applicable.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Authors’ contribution
All authors contributed equally to the drafting, writing, sourcing, article screening and final proofreading of the manuscript.