Volume 5, Issue 6
June 2025
Diabetic Ketoacidosis in Pediatric Patients: Fluid Management and Cerebral Edema Risk
Abdulghani Abdulaziz Alzamzami, Duaa Ahmed Alshab, Mithaq Abdullah Alrabiea, Fidaa Saleh Al Mohammed, Saad Fudail Albuainain, Ola Ali Mallawi, Rashed Saleh Alfehaid, Abdullah Tariq Almalki, Abdulrahman Abrahem Bagber, Marwan Abdulmoomin Sabbagh, Khalid Fayez Alotaibi
DOI: http://dx.doi.org/10.52533/JOHS.2025.50605
Keywords: diabetic ketoacidosis, glucose, cerebral edema, insulin, fluid therapy
Diabetic ketoacidosis represents a critical and potentially life-threatening complication associated with diabetes, predominantly encountered within pediatric populations. This condition is characterized by three key metabolic derangements: hyperglycemia, metabolic acidosis, and the presence of ketone bodies in both blood and urine. The pathophysiology of diabetic ketoacidosis is primarily driven by an absolute or relative deficiency of insulin, which subsequently triggers the secretion of counter-regulatory hormones, including glucagon, cortisol, growth hormone, and catecholamines. These hormonal responses facilitate processes such as gluconeogenesis, lipolysis, and ketogenesis, which collectively contribute to significant metabolic disturbances. A hallmark of diabetic ketoacidosis is the accompanying fluid depletion, which significantly exacerbates morbidity and mortality rates associated with the condition. Pediatric patients are at an increased risk of developing cerebral edema, an often-fatal complication, due to immature cerebral autoregulatory mechanisms. This review article meticulously examines the pathophysiological mechanisms, clinical manifestations, diagnostic criteria, and global prevalence of Diabetic ketoacidosis among children. The critical imperative for early identification and careful differentiation from hyperglycemic hyperosmolar state, particularly in atypical clinical presentations, is emphasized. The article provides a comprehensive overview of contemporary fluid management protocols, advocating for initial resuscitation with isotonic saline and outlining ongoing rehydration strategies tailored to individual weight or body surface area. It also underscores the necessity of vigilant monitoring of serum sodium concentrations, as these levels may appear elevated relative to declining serum glucose, erroneously suggesting hypernatremia. Furthermore, the review delves into the pathogenesis and clinical trajectory of cerebral edema in pediatric Diabetic ketoacidosis, evaluating evidence-based interventions such as intravenous mannitol and hypertonic saline. Recommendations for proactive monitoring and early treatment strategies are presented to mitigate neurologic damage and enhance patient outcomes. Given that pediatric Diabetic ketoacidosis continues to pose a significant global health challenge, targeted education, prompt diagnosis, and meticulous management are essential in reducing its associated complications.
Introduction
Diabetic ketoacidosis occurs when the venous pH is less than 7.3, the serum bicarbonate is less than 15 mmol/L, and the serum glucose concentration is more than 11 mmol/L, in addition to the presence of conditions such as ketonuria, ketonemia, and glucosuria. In mild diabetic ketoacidosis, the venous pH is between 7.2 and 7.3, and the serum bicarbonate is less than 15 mmol/L. In moderate diabetic ketoacidosis, the venous pH is between 7.1 and 7.2, and the serum bicarbonate is less than 10 mmol/L, whereas, in cases of severe diabetic ketoacidosis, the venous pH is less than 7.1, and the serum bicarbonate is less than 5 mmol/L (1). Diabetic ketoacidosis results from absolute or relative insulin deficiency, which results in intracellular starvation in tissues that depend mainly on insulin, such as the muscles, liver, and adipose tissue (2). This starvation stimulates the release of counter-regulatory hormones such as the growth hormone, catecholamines, glucagon, and cortisol (2). These counter-regulatory hormones stimulate hepatic and renal glucose production in addition to oxidation of fatty acids to ketone bodies, lipolysis and proteolysis (3). However, during fasting, ketone bodies are not formed due to the absence of the citric acid cycle, which processes glucose and turns it into ketone bodies (3). The pathophysiology of diabetic ketoacidosis is similar in adults and children. However, they differ in certain aspects. Approximately 15-20% of infants in developed countries who are newly diagnosed with diabetes suffer from diabetic ketoacidosis (4). On the other hand, the rates of diabetic ketoacidosis are inversely proportional to the rates of diabetes. Additionally, in the United States, the rates of diabetic ketoacidosis at the time of diagnosing diabetes are approximately 25% (5). The prevalence of diabetic ketoacidosis decreases with age. For instance, the prevalence of diabetic ketoacidosis in children aged five years is about 36%, whereas it represents 16% in children of 14 years of age (5). For instance, the younger the child, the more difficult it is to diagnose them with diabetic ketoacidosis. Infants and toddlers are usually misdiagnosed as suffering from pneumonia, bronchitis, and asthma because it is difficult to identify weight loss, polyuria, and polydipsia (4). This misdiagnosis leads to mistreating the infants and children with glucocorticoids, which aggravate the metabolic derangements (4). Additionally, misdiagnosing infants and children leads to acidosis and dehydration, reduced consciousness and coma (4). The degree of dehydration expresses the loss of total body weight. Therefore, the amount of fluid per kilogram decreases as the child grows (5). Cerebral mechanisms are not well developed, and the autoregulatory systems are not mature in children. These factors render the child prone to cerebral edema. Cerebral edema occurs in 0.5% to 1% of children suffering from diabetic ketoacidosis, which is the most common cause of mortality in children, accounting for 60% to 90% (6-9). The mortality rates in children with diabetic ketoacidosis range from 0.15% to 0.3%. However, when cerebral edema develops, the mortality rate increases to a range between 20% and 25% (4). If the child survives diabetic ketoacidosis and cerebral edema, 10 –25% of them will suffer from pituitary insufficiency (4). This review article discusses diabetic ketoacidosis, highlighting the fluid management and cerebral edema risk.
Methodology
This narrative review is based on a comprehensive literature search conducted on April 4, 2025, using Dynamed, ScienceDirect, PubMed, Wiley Library, MDPI, Oxford Academic, BMC, and Cochrane databases. The research utilized Medical Subject Headings (MeSH) terms and relevant keywords, such as Diabetic Ketoacidosis in Pediatric Patients, to identify studies that examined fluid management of diabetic ketoacidosis in pediatric patients. A manual search was also conducted using Google Scholar, and the reference lists of identified papers were reviewed to locate additional relevant studies. No restrictions were applied regarding publication date, language, participant age, or type of publication, ensuring a broad and inclusive exploration of the available literature.
Discussion
Pathophysiology of Diabetic Ketoacidosis in Pediatric Patients
Absolute insulin deficiency or infection, stress, or insulin insufficiency are factors that stimulate the release of counter-regulatory hormones. Counter-regulatory hormones stimulate gluconeogenesis and glycogenolysis and reduce glucose utilization, which results in hyperglycemia (serum glucose concentration becomes about 11 mmol/L), electrolyte loss, osmotic diuresis, dehydration, hyperosmolarity, and decreased glomerular filtration. Additionally, the lipolysis process provides free fatty acids. When fatty acids are oxidized, they facilitate gluconeogenesis and produce ketone bodies. Ketone bodies cause metabolic acidosis (Figure 1).
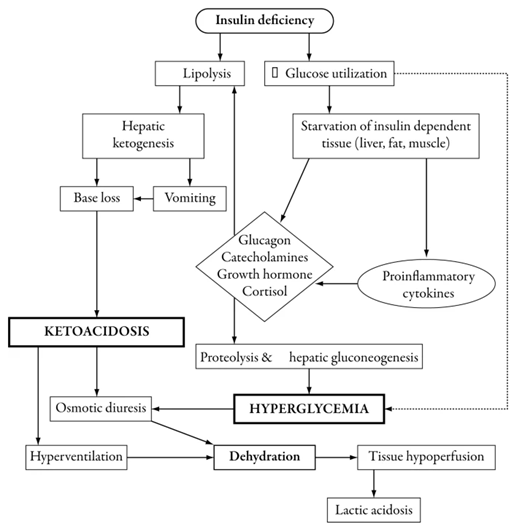
Figure 1: Pathophysiology of diabetic ketoacidosis in children (1).
The clinical manifestations of diabetic ketoacidosis are dehydration, polyuria, polydipsia, deep gasping respiration (Kussmaul breathing) to reduce the partial pressure of carbon dioxide and buffer the acidosis, and reduced consciousness, which can progress to coma. The onset and frequency of DKA vary widely across geographic regions. The frequency of diabetic ketoacidosis in Europe, North America, and Australia ranges from 15% to 70% (5, 8-16). For instance, in Europe, the frequency of diabetic ketoacidosis ranges between 11% and 67%. Whereas the frequency in Australia, between 1985 and 2000, was 26% (1). In New Zealand, the frequency of diabetic ketoacidosis was 63% in 1988 and 1989, then it declined to 42% in 1995 and 1996 (17). Diabetic ketoacidosis is particularly common in children under five years of age and in those from socioeconomically disadvantaged backgrounds with limited access to healthcare services (14, 18-20). Factors such as lower parental education levels, lack of health insurance, and low household income are associated with higher DKA rates in children (20). Among children and adolescents diagnosed with type 1 diabetes, the prevalence of diabetic ketoacidosis is estimated to be approximately 1 to 10 per 100 individuals (4). For instance, in Colorado, diabetic ketoacidosis was present at diagnosis in 28.4% of cases. The incidence among uninsured children was 6.2 times higher than among those with insurance, with the uninsured group also experiencing more severe episodes (20).
Long-term reductions in the incidence of diabetic ketoacidosis at onset have been achieved through educational initiatives targeting both healthcare providers and the general public (1). While DKA is commonly associated with type 1 diabetes, approximately 25% of pediatric DKA cases are now attributed to type 2 diabetes (21). Maniatis et al. conducted a study on 1243 patients in Colorado (20). They found that about 20% of the patients suffered from recurrent episodes of diabetic ketoacidosis, accounting for 80% of the episodes experienced by all the patients in the study. Recurrent diabetic ketoacidosis is linked to poor metabolic control, female gender, previous episodes of diabetic ketoacidosis, psychiatric disorders such as eating disorders, absence of medical care, or insulin pump therapy (20). Since insulin pumps deliver only rapid- or short-acting insulin, any interruption in delivery can quickly lead to insulin deficiency and precipitate DKA. However, education programs targeting both patients and caregivers on proper insulin pump use and addressing misuse significantly reduced the recurrence of DKA episodes (22, 23).
Diagnosis and management
Hyperglycemic hyperosmolar state is a state of the body when serum glucose is more than 600 mg/ml, serum osmolality is greater than 320 mOsm/L, and there is minimal ketonemia or ketonuria. Hyperglycemic hyperosmolar state is found in type 2 diabetes patients. However, it can lead to misdiagnosis of the case as diabetic ketoacidosis (24). In contrast, the manifestation of hyperglycemic hyperosmolar state in individuals with type 1 diabetes may suggest that the patient ingested glucose-rich fluids to satisfy excessive thirst associated with polydipsia (25). Hyperglycemic hyperosmolar state is associated with high mortality rates (1). Body dehydration is calculated as 5% if the skin elasticity is reduced, there is evidence of tachycardia and deep breathing, and the mucous membranes are dry. If the capillary refill time is greater than three seconds, then the body dehydration is estimated as 10%. Additionally, the level of consciousness is measured using the Glasgow Coma Scale, and venous blood tests are performed to measure serum glucose, pH, urea nitrogen, electrolytes, creatinine, osmolality, hemoglobin, and ketone bodies (1).
Diabetic ketoacidosis causes severe depletion of water and fluids, affecting intracellular and extracellular fluids. The dehydrated patient continues to exert urine output until the glomerular filtration and renal blood flow are affected. The magnitude of dehydration depends on several factors, including the severity and duration of illness, the components of the food and fluids consumed before seeking medical care, and the duration during which the patient was able to maintain electrolyte and fluid intake (4). The hydration estimate in pediatric patients is 5% to 10%. Fluid deficit estimate in moderate cases ranges between 5% and 7%, while the estimate in severe cases is 10%. Children rarely experience shock during a diabetic ketoacidosis episode. Diabetic ketoacidosis pediatric patients suffering dehydration should have their osmolality in the 300- to 350-mosm/l range. The extracellular fluid contraction indicators are increased serum urea nitrogen and hematocrit. However, the serum sodium concentration is not a reliable indicator because the presence of glucose in the extracellular fluid causes an osmotic movement of water into the extracellular space, resulting in dilutional hyponatremia in addition to the low sodium content in diabetic ketoacidosis due to the increased lipid friction (4). However, it is crucial to monitor sodium levels following the initiation of fluid therapy, as a decrease in serum glucose is typically accompanied by an increase in sodium concentration. The goal of introducing fluid therapy is to restore the volume of blood circulation, glomerular filtration, to replace the sodium, extracellular fluid, and intracellular fluid water deficit, and to eliminate glucose and ketones from the blood circulation (4). It is crucial to avoid administering excessive amounts of fluids to avoid developing cerebral edema. During the first 1 to 2 hours of fluid resuscitation, 10 to 20 mL/kg of 0.9% sodium chloride (NaCl) should be administered to restore peripheral perfusion. Ongoing fluid requirements can be calculated using either of the two standard methods. The first method is administering 1000 mL for the first 10 kg of body weight, plus 500 mL for the next 10 kg, and an additional 20 mL/kg for each kilogram above 20 kg; the second method is administering 1500 mL per square meter of body surface area (1). Then the remaining replacement fluid can be administered over the remaining 22 or 23 hours. In mild to moderate cases, fluid that was taken orally at home or in the emergency room can be included in the fluid calculation. Conversely, in severe cases, oral administration of fluids should be initiated before 24 hours (1). However, the urinary output should not be considered in the fluid requirement except in the case of hyperglycemic hyperosmolar state. After administering 0.9% of NaCl in the first 24 hours, 0.45% of NaCl should be administered for rehydration and maintenance. The sodium concentration will increase to the corrected level as the serum glucose concentration decreases (1). To prevent a rapid drop in blood glucose levels, glucose should be added to the IV fluids once serum glucose falls to approximately 300 mg/dL. This is often managed using the “two-bag system,” in which two IV fluid bags contain the same concentrations of sodium and potassium, but only one contains 10% dextrose. This approach allows clinicians to adjust the glucose concentration as needed without altering electrolyte delivery (26).
Cerebral edema
Pediatric patients suffering from diabetic ketoacidosis are at risk of developing cerebral edema. Elevated serum urea nitrogen, acidosis, and hypocapnia are associated with diabetic ketoacidosis and are risk factors for cerebral edema. The pediatric brain is particularly vulnerable to the metabolic and vascular changes resulting from diabetic ketoacidosis. Cerebral edema that is associated with diabetic ketoacidosis is rarely found in patients who are older than 21 years or in adults in a hypoglycemic hyperosmolar state (1).
Several studies have reported a connection between cerebral edema and failure of sodium concentration to increase to the corrected levels in diabetic ketoacidosis cases. This connection can be attributed to the alterations that occur in the brain, affecting the antidiuretic hormone secretion (6, 27). In a study of 61 pediatric patients suffering cerebral edema, which found that patients with the worst outcomes were the ones with elevated serum urea nitrogen and pressure carbon dioxide less than 22 mmHg (6, 28). Cerebral edema refers to an accumulation of water within the cerebral tissue, leading to an increase in tissue volume. The cerebral edema can be vasogenic due to a breakdown in the blood-brain barrier, cytotoxic due to metabolic derangement, or osmotic due to hypernatremia (Figure 2). However, there is no evidence explaining either the mechanism or the location of cerebral edema. Two-thirds of the patients develop cerebral edema after 6 to 7 hours after initiation of the treatment, and the rest develop cerebral edema after 10 to 24 hours (6, 27-28).
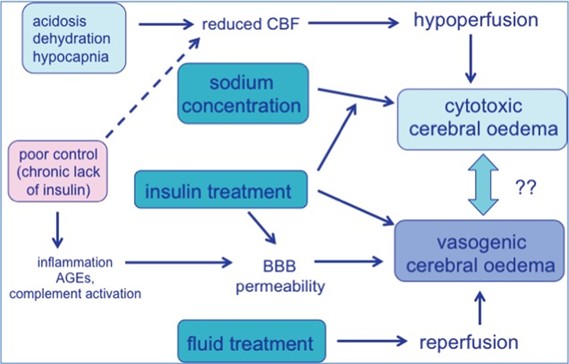
Figure 2: Pathophysiology of cerebral edema (29).
The initial brain CT was taken 2 to 44 hours after diagnosing the subjects with cerebral edema. They found that 39% of the subjects showed no abnormalities, 26% had diffuse edema, and three of them had hemorrhage as well, 17% had either intraventricular or subarachnoid hemorrhage, and 22% of them had focal brain injury (27). Reports have shown that cerebral edema is associated with high mortality and morbidity rates. Rosenbloom et al. (30) conducted a study on 69 cases and found that the mortality rate was 64%, survival without disability was 14%, disability without excluding dependence was 9%, and severe disability was in 13% of the subjects. Additionally, a report conducted in Canada found that 19% of the patients had cerebral edema before initiation of diabetic ketoacidosis (1, 30).
Cerebral oedema is treated by administering IV mannitol with a dosage of 1g/Kg for 20 minutes and repeat the process when necessary for 1 to 2 hours. This treatment is initiated as soon as the cerebral edema is diagnosed. Mannitol lowers blood viscosity and hematocrit and improves cerebral blood flow, oxygenation, and vasoconstriction in parts of the brain with integral autoregulation and red cell deformability. It also has a direct osmotic effect as it reduces the extracellular free water. However, some reports stated that mannitol can cause rebound edema and renal failure if used for a prolonged period. Therefore, hypertonic saline is established as the standard for treating acute intracranial hypertension in head injury. Hypertonic saline is as effective as mannitol (1). Hypertonic saline was first used in a 13-year-old who was diagnosed with diffuse cerebral edema. When she started receiving mannitol as a treatment for cerebral edema, she developed a headache after 20 minutes. Therefore, she was administered 5 mL/kg of 3% saline. After five minutes, she woke up with a recovered neurologic function. Therefore, it is recommended to switch to using 5–10 mL/kg 3% saline if the patient was not responsive to 1g/Kg of mannitol (31). Additionally, if the respiratory system of the patient is compromised, then intubation should be reversed (28).
Conclusion
Pediatric diabetic ketoacidosis constitutes a critical clinical emergency that necessitates immediate and meticulous management. An in-depth understanding of its pathophysiology and associated risk factors significantly enhances prevention efforts, particularly in vulnerable pediatric populations. Administration of fluid therapy must be approached with caution to effectively restore hemodynamic stability while mitigating the risk of precipitating cerebral edema. Furthermore, the implementation of comprehensive education and support programs for both families and healthcare providers play a vital role in reducing the incidence and recurrence of diabetic ketoacidosis, along with its potentially life-threatening complications.
Disclosures
Author contributions
The author has reviewed the final version to be published and agreed to be accountable for all aspects of the work.
Ethics statement
Not applicable.
Consent for publications
Not applicable.
Data availability
All data is provided within the manuscript.
Conflict of interest
The author declares no competing interest.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Acknowledgements
Not applicable.