Volume 4, Issue 11
November 2024
Bacterial Aspects of Contact Lenses: Symptoms, Diagnosis, and Treatment: A Review Article
Mohammed Abdulrahman Alem
DOI: http://dx.doi.org/10.52533/JOHS.2024.41103
Keywords: contact lenses, eye infection, corneal ulcers, corneal scraping, bacterial keratitis, eye treatment
Contact lenses represent one of the common methods of vision correction. Estimates suggest that over 35 million people in the United States (US) alone use contact lenses for various reasons, such as correcting astigmatism, myopia, hyperopia, presbyopia and even cosmetic. It has been widely reported that contact lens-related microbial keratitis is the predominant cause of infectious keratitis in numerous regions, including North America, Europe, Australia, the Middle East, and the Far East. Adhesion and growth of bacteria on the surface of contact lenses have been linked to inflammation of the cornea and corneal ulcers, which are open sores on the cornea. As the cornea is the transparent front covering of the eye, a corneal ulcer can obstruct vision and lead to permanent blindness; therefore, it should be treated immediately. Understanding the bacterial aspects of contact lens use is crucial for both healthcare professionals and contact lens wearers. The aim of this research is to review and evaluate the available information in outline of bacterial keratitis related to contact lenses, including symptoms, diagnosis, along with a comprehensive overview of microbiological laboratory techniques in bacterial characteristics that can help determine the appropriate treatment for a contact lens-associated infection to prevent or reduce the need for surgery in patients with severe corneal ulcers.
Introduction
The bacterial aspects of contact lenses refer to the role that bacteria play in contaminating contact lenses, potentially leading to eye infections. When contact lenses are not handled, cleaned, or stored properly, bacteria can adhere to the lens surface or accumulate in the lens case. Contact lenses have been used as glasses since time immemorial, and now contact lenses are being widely used for optical as well as cosmetic purposes (1). Globally, it is estimated that the number of contact lens wearers has increased, not only in adults but also in pediatrics (2, 3). The increased interest in contact lenses among the pediatric and geriatric populations, as well as in the youth, has created a need for increased awareness and understanding of the associated complications.
One of the major factors contributing to complications and failures in the use of contact lenses is the transmission of pathogenic microorganisms. The transmission of pathogenic microorganisms significantly contributes to complications in contact lens use, often leading to severe ocular infections. Among these, bacterial keratitis is the most common and serious condition associated with improper lens handling and hygiene (4). In addition to bacterial keratitis, more severe complications, such as orbital cellulitis and dacryocystitis, may develop if bacteria spread from the ocular surface to deeper tissues. Advanced infections can result in significant health consequences, including vision loss and systemic infections if not treated promptly.
Contact lens use can also induce notable changes in the ocular surface, such as alterations in tear film composition and the onset of dry eye symptoms. Commonly reported effects include reduced blink rates, prolonged upward eye movements with closed eyelids, and diminished tear clearance, which are often attributed to lens tightness and reduced oxygen availability (5). Research has indicated that the protective tear film may become compromised when contact lenses are worn (6)
Key risk factors associated with contact lens use include improper handling and environmental exposure, both of which can increase bacterial colonization on lenses and heighten the risk of infections like bacterial keratitis (7). Overnight contact lens wear significantly reduces oxygen supply to the cornea, compromising its structural integrity and increasing its susceptibility to bacterial infection. Therefore, proper lens handling, storage, and adherence to recommended wearing schedules are essential for mitigating these risks.
Moreover, hygiene plays a critical role in preventing complications. The corneal surface becomes more vulnerable during sleep, leading to increased water retention and accelerated breakdown of the epithelial layer (8, 9). Poor hygiene practices, such as using contaminated lens solutions or improperly applying cosmetic lens, further elevate the risk of severe conditions like corneal ulcers and conjunctivitis, which may result in permanent vision loss if left untreated (10, 11). Delayed recognition and treatment of infections can lead to poorer prognoses for patients.
The prevalence of microbial contamination among contact lens wearers is well-documented, with improper handling and inadequate hygiene practices significantly contributing to bacterial colonization. Incidence rates of bacterial keratitis, a severe complication associated with contact lens use, range from 4 to 21 cases per 10,000 wearers annually across various populations (12, 13). These findings underscore the critical importance of strict adherence to hygiene protocols in reducing the risk of severe complications, while timely medical intervention plays a pivotal role in preventing serious clinical outcomes. Rigorous hygiene practices significantly lower infection risks, and early treatment is crucial for enhancing prognostic outcomes.
This review aims to provide an in-depth analysis of complications corneal ulcers, with a particular focus on bacterial keratitis associated with contact lens misuse. By examining the regional variations in microbial profiles and the role of bacterial biofilm in infection persistence and severity, this review will assess the clinical implications of these infections. Diagnostic approaches such as corneal scraping and advanced laboratory techniques for pathogen identification will be discussed, along with an exploration of the symptoms and complications that arise from corneal ulcers. Furthermore, this review will evaluate current management strategies and highlight the critical need for adopting preventive measures to reduce the risks associated with contact lens wear. Ultimately, the findings presented here will not only inform future research but also contribute to the development of more effective guidelines for the prevention and management of contact lens-related bacterial keratitis and corneal ulcers.
Methods
The scope of this review was defined to address key research questions related to corneal ulcers and bacterial infections in contact lens wearers. These questions included identifying the primary bacterial pathogens involved, the characteristic symptoms of such infections, the diagnostic methods employed in clinical practice, and the current treatment protocols used in their management.
A systematic search of academic databases was conducted to gather relevant studies: PubMed was used to retrieve articles on clinical aspects, including symptoms, diagnosis, and treatment of bacterial infections in contact lens wearers. The Web of Science was employed to access broader scientific literature, particularly in the fields of microbiology and epidemiology.
A combination of specific and broad search terms was utilized to capture relevant literature. Keywords included: 'Corneal ulcers' AND 'Bacterial infections' AND 'Contact lenses,' 'Pseudomonas aeruginosa' AND 'Contact lens keratitis,' 'Diagnosis' AND 'Bacterial keratitis' AND 'Contact lenses,' 'Symptoms' AND 'Conjunctivitis' AND 'Contact lens use,' and 'Antibiotic treatment' AND 'Microbial keratitis' AND 'Contact lenses.' The search was restricted to studies published in the last 10 years, up to March 2024, to ensure that the most recent research and clinical practices were included.
Inclusion Criteria: Peer-reviewed articles written in English were included, along with studies specifically addressing bacterial infections related to contact lens use. Clinical trials, systematic reviews, and meta-analyses focusing on symptoms, diagnosis, and treatment were also included.
Exclusion Criteria: Studies focusing on non-bacterial pathogens (e.g., fungal or viral infections), articles that did not specifically address symptoms, diagnosis, or treatment of bacterial infections, and non-peer-reviewed sources or studies lacking methodological rigor were excluded.
Data extraction focused on several key areas, for bacterial pathogens the distribution of microorganisms isolated from contact lens-related microbial keratitis cases was analyzed, with an emphasis on identifying major bacterial species. Symptoms common symptoms reported across studies, such as corneal ulcers, eye pain, redness, discharge, and blurred vision, were analyzed. Diagnostic methods, Information on diagnostic techniques, including clinical examination, microbiological cultures, corneal scraping, and imaging methods were extracted and reviewed. Treatment Protocols, the types of antibiotic treatments used their duration, and their reported success rates were reviewed, with a focus on commonly used antibiotics.
The review prioritized randomized controlled trials, observational studies, and case series to provide a comprehensive and evidence-based synthesis of the current literature on bacterial infections in contact lens wearers.
Review
Corneal Ulcers
The eye’s cornea has several layers of cells on it, much like the skin. The top layer is the epithelium, which prevents germs from entering the eye. Although it heals quickly, the epithelium can be scraped off by any injury (4, 14). The surface cells are tightly packed together with no empty areas in between, as in the skin. The cells are attached to a membrane that does not allow new cells to grow through it. If the surface cells are lost, healing cells have to travel (or “slide”) over from the side. This action prevents microorganisms from entering the cornea and causing infection by growing behind the healing epithelium the “roof” of the wound (15). On the other hand, the deeper layers of the cornea have cells that reproduce and grow, unlike on the surface. These cells are lost slowly as the ulcer goes deeper; they leave a “crater,” or lesion, on the corneal layers (16). This can occur in the place of infection. If the cornea is dried out, it may become inflamed with the condition called keratitis. Keratitis is inflammation of the cornea. It is marked by pain, decreased visual potential, and ocular inflammation. Although keratitis is common, it can be hard to diagnose because it masquerades as other conditions (4, 17). Keratitis can be caused by various factors such as injury, poorly fitting hard or soft contact lenses, or infection by bacteria, fungi, or viruses. The disease has the potential to cause severe visual loss due to corneal scarring, and it is the leading cause of blindness. Chronic keratitis can lead to the loss of the eye itself. Since the eye is a cosmetically and psychologically important part of the body, this can be especially traumatic for the patient. The management of corneal ulcers has been simplified by the ability of the doctors to use cyanoacrylate glue on small corneal perforations. This rapidly seals the defect, relieves pain, and offers faster visual recovery than historically encountered with bandaged contact lenses (18).
A corneal ulcer is an open sore on the cornea, which is the clear frontal surface of the eye (19). It covers the iris and the round pupil, much like a watch’s crystal covers the face of the watch. The cornea also acts as a filter, screening out some of the most damaging ultraviolet wavelengths in sunlight (20). It is a very important part of sight. When the surface of the cornea is disturbed, it cannot heal on its own, leaving the eye vulnerable to serious infection and the possibility of permanent visual loss. There are several infections and conditions that can cause corneal ulcers and an ulcer may rapidly penetrate the cornea.
Regional bacterial keratitis
The primary bacterial agents implicated in contact lens-related corneal ulcers are Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae (21, 22). (Table 1) presents a detailed overview of microorganism distribution in contact lens-associated microbial keratitis across diverse global regions, illustrating variations likely influenced by regional environmental factors and hygiene practices.
In the Middle East, particularly in Riyadh, Saudi Arabia, microbial keratitis profiles reveal an evenly distributed incidence of Pseudomonas spp. and S. aureus, each comprising 21% of cases. Additionally, 43% of isolates in Riyadh are Gram-positive bacteria, highlighting the diversity of microbial threats within this area (38). In Dhahran, Saudi Arabia, Pseudomonas spp. is notably prevalent, accounting for 58% of cases, which indicates a significant risk factor associated with this pathogen in the region. S. aureus appears in 17% of samples, potentially reflecting specific hygiene practices or environmental influences on bacterial profiles. The presence of both Gram-negative and Gram-positive bacteria, each at 17%, suggests a mix of bacterial sources in this area, whether from patient populations or environmental contamination (28). This microbial pattern may be attributed to the region’s dry climate and particular contact lens hygiene routines, which may influence the spectrum of microorganisms.
In the United States, Pseudomonas spp. is highly represented in various regions, including Florida (46%), South Texas (58%), California (44%), and South Texas (58%). Florida also shows a substantial rate of S. aureus (48%), while California reports an absence of S. aureus and Streptococcus spp., suggesting unique environmental impacts across states. Warmer and more humid climates in South Texas and Florida may contribute to the growth and persistence of Pseudomonas spp. and Gram-negative bacteria, indicating a distinct regional microbial profile associated with keratitis in these areas (29, 36, 39).
|
Table 1. Distribution of various microorganisms isolated from contact lens-related microbial keratitis cases in different regions |
|||||||
|
Microorganisms % |
P. spp |
S. aureus |
Strept. spp |
G. N |
G. P |
Other |
Reference |
|
Adana, Turkey |
21 |
0 |
0 |
20 |
16 |
43 |
(23) |
|
Ahwaz, Iran |
80 |
12 |
0 |
8 |
0 |
0 |
(24) |
|
Australia |
36 |
0 |
0 |
18 |
14 |
32 |
(19) |
|
Babol, Iran |
79 |
14 |
0 |
0 |
7 |
0 |
(25) |
|
Brazil |
25 |
22 |
7 |
5 |
23 |
18 |
(26) |
|
China |
43 |
0 |
0 |
33 |
0 |
24 |
(27) |
|
Dhahran, KSA |
58 |
17 |
0 |
8 |
17 |
0 |
(28) |
|
Florida, USA |
46 |
48 |
0 |
0 |
0 |
6 |
(29) |
|
France |
75 |
0 |
0 |
9 |
15 |
0 |
(30) |
|
Germany |
40 |
0 |
0 |
52 |
8 |
0 |
(31) |
|
India |
74 |
0 |
0 |
5 |
7 |
14 |
(32) |
|
Isfahan, Iran |
5 |
0 |
0 |
15 |
72 |
8 |
(33) |
|
Izmir, Turkey |
43 |
0 |
0 |
20 |
13 |
24 |
(34) |
|
Korea |
42 |
0 |
0 |
58 |
0 |
0 |
(35) |
|
California, USA |
44 |
0 |
0 |
6 |
33 |
17 |
(36) |
|
Netherland |
69 |
0 |
0 |
0 |
0 |
31 |
(37) |
|
Portugal |
90 |
0 |
0 |
10 |
0 |
0 |
(22) |
|
Riyadh, KSA |
21 |
21 |
0 |
0 |
43 |
15 |
(38) |
|
South Texas, USA |
58 |
7 |
4 |
22 |
9 |
0 |
(39) |
|
Sweden |
16 |
12 |
9 |
7 |
56 |
0 |
(40) |
|
UK |
26 |
0 |
2 |
46 |
27 |
0 |
(41) |
P. spp: Pseudomonas species, S. aureus: Staphylococcus aureus, Strep. spp: Streptococcus species, G.N: Gram Negative bacteria, G. P: Gram positive bacteria
In South America, data from Brazil demonstrate a relatively balanced distribution of Pseudomonas spp. (25%), S. aureus (22%), and other microorganisms, indicating a multifactorial microbial environment. Brazil’s tropical and subtropical climates, combined with specific cultural practices related to contact lens hygiene, likely foster a range of microorganisms capable of causing keratitis. This diverse microbial distribution suggests that, in regions like Brazil, public health initiatives that target a broad range of pathogens may be most effective in reducing the incidence of keratitis among contact lens users (26).
In Europe, substantial regional differences emerge. For example, in Portugal, Pseudomonas spp. predominates, representing 90% of keratitis cases, suggesting that environmental factors or local lens care habits may favor this pathogen. Such distributions are likely shaped by Europe’s temperate climates and specific water quality factors, which support a unique spectrum of microorganisms (22).
The microorganism prevalence survey across various regions reveals distinct microbial profiles, underscoring both the diversity and regional dominance of certain bacteria in ocular infections. In the United Kingdom, Gram-negative bacteria are the most commonly isolated (46%), likely associated with environmental or lens-related factors favoring these bacteria. Pseudomonas spp. also features prominently at 26%, while Streptococcus spp. account for a minor 2%, suggesting that regional healthcare practices and climate conditions may influence pathogen prevalence (41). Germany presents a unique profile, with a high incidence of Gram-negative bacteria (52%) and Pseudomonas spp. (40%), while lacking other significant bacterial species. This predominance suggests that environmental or healthcare-associated factors may strongly influence microbial patterns, and the lack of Gram-positive and Streptococcal bacteria may reflect regional infection control measures or reduced exposure to these pathogens (31).
In France, Pseudomonas spp. overwhelmingly dominates the microbial landscape at 75%, one of the highest rates observed, indicating a possible environmental reservoir or specific regional predisposition for this organism. Only 9% of cases contain Gram-negative bacteria and 15% Gram-positive bacteria, suggesting a concentrated microbial profile with Pseudomonas spp. as the primary pathogen in ocular infections (30). Sweden, however, displays a diverse microbial landscape, with Gram-positive bacteria making up 56% of cases, the highest in the survey. This includes notable proportions of S. aureus (12%) and Streptococcus spp. (9%), and a low incidence of Gram-negative bacteria (7%), which may result from Sweden’s cooler climate, healthcare protocols, or hygiene practices that favor Gram-positive bacteria in ocular infections (40).
In Asia, the prevalence of Pseudomonas spp. is particularly high in India (74%) and, in some cases, in Iran (up to 80%), likely influenced by tropical and semi-arid climates conducive to microbial persistence. India’s humid climate favors bacteria such as Pseudomonas spp., while in Iran, data reveal a high prevalence of both Pseudomonas spp. and Gram-positive bacteria (up to 72% in some cases), suggesting that climate and lens handling practices impact microbial profiles associated with keratitis(24,25,32,33). In Korea, Gram-negative bacteria account for 58% of cases, with the remaining 42% comprising Pseudomonas spp., and an absence of Gram-positive or Streptococcal bacteria (35). This microbial pattern may reflect environmental exposures, healthcare protocols, or demographic factors specific to the region, emphasizing a focus on Gram-negative bacteria in infection prevention and treatment strategies.
In Australia, a balanced microbial distribution across Pseudomonas spp., Gram-negative, and other microorganisms suggest an environment that supports diverse microbial populations. Australia’s varied climates, from tropical to temperate, likely contribute to this diversity. The substantial presence of other microorganisms, which may include fungi and opportunistic organisms, indicates that the local climate supports a broad range of pathogens specific to Australian conditions (19).
These observations highlight the global variability in pathogen prevalence, emphasizing the need for regionally tailored infection control strategies and treatment approaches that consider distinct microbial patterns. Variables such as climate, healthcare infrastructure, hygiene practices, and contact lens usage likely contribute to these differences. The data provided serves as an invaluable resource for ophthalmologists, microbiologists, and public health officials, offering critical insights into microbial risks associated with contact lens wear. Ongoing surveillance and research remain essential to addressing emerging microbial threats and ensuring effective management of keratitis cases worldwide.
Misuse of contact lenses
The misuse of contact lenses is a significant contributing factor to the development of corneal ulcers. Many cases arise from practices such as wearing contact lenses overnight, which can create an environment conducive to bacterial growth and compromise corneal health. Additionally, poor hygiene practices, including inadequate hand washing before handling lenses or failing to clean and store them properly, increase the risk of infections. Furthermore, the use of inadequate cosmetic hygiene practices can introduce harmful pathogens to the eye, exacerbating the risk of keratitis and other complication (11, 42). In cases in which the corneal epithelium is disorganized, these bacteria can penetrate the stroma of the cornea and trigger irritation (43). Various factors exacerbate this irritation, ranging from a lack of cornea oxygen to the changes in the corneal microenvironment, both of which are direct results of wearing contact lenses. The cornea receives nutrients and oxygen from the air and from tears film multiple factors (9). The risk of developing an infected corneal ulcer with overnight contact lenses of any type has been shown to be greater than that for normal daily wear (11, 44). This is mainly due to the fact that microorganisms adhere more easily to the surface of a contact lens, and that overnight the lens acts as a barrier to the natural immune defenses of the cornea.
When bacteria infect the eye and cause severe inflammation, the corneal layer may be infiltrated. This increases the chance of developing corneal scars, which hamper the usual quality and transparency of the corneal layer, leading to clouded vision. In more severe cases, corneal infiltration and the bacteria may interact in complex ways. If we fail to control an infection, the corneal layer will be thinned, resulting in a greater corneal curve. Keratitis production can also cause corneal layer infiltration. This might result in an eye rupture because the corneal layer becomes unable to resist the internal pressure of the aqueous humor or fluid inside the eye.
In a recent investigation employing molecular techniques, it was established that bacterial biofilm adheres to around 75% of contact lenses (45). A biofilm is an intricate accumulation of microorganisms on a solid surface. One of the defining aspects of biofilm is the microorganisms’ adhesion to the surfaces they colonize, as well as to one another, and their frequent inclusion in a matrix of extracellular polymer substances, which they produce themselves when colonizing contact lens. It is thus advisable in such cases to immediately cease wearing contact lenses and schedule an appointment with a medical professional to undergo an evaluation and learn about suitable procedures for recovery (46).
The treatment of corneal ulcers is essential to maintaining vision and ocular health. Initial diagnosis, typically through corneal scraping and microbial culture, informs the application of targeted therapies. Bacterial ulcers are usually treated with broad-spectrum topical antibiotics, adjusted according to sensitivity results; other infections require antifungal or antiviral medications. Additional treatments involve pain management, anti-inflammatory drugs, sometimes corticosteroids and preventive measures such as stopping contact lens use and addressing underlying conditions to avoid recurrence. Severe cases may necessitate surgical procedures for restoring ocular integrity and function.
Symptoms of Corneal Ulcers
Conjunctival injection
Patients with corneal ulcers often present with eye redness, also known as conjunctival injection. In this condition, the small blood vessels become dilated and inflamed, turning the sclera red or pink. Eye redness caused by corneal ulcers may be mild or severe. To detect mild eye redness, the clinician reviewed the patient's medical history and used a bright light to closely examine the symptoms, ensuring accurate assessment and diagnosis. (47). A medical examination should distinguish between simple eye redness and redness related to corneal ulcers. For mild eye redness, the clinician will use a slit lamp to visualize the cornea. This is crucial in detecting more subtle forms of corneal ulceration.
Eye Pain
Corneal eye pain is derived from the cornea. The latter contains millions of nerves, which is one of the reasons corneal eye pain can be so severe; any damage to or inflammation of the nerves on the cornea can cause significant pain. In addition to nerve-related pain, however, corneal eye pain can also occur due to other conditions, such as inflammation, dryness, or surface irregularities (48). This means that there are many different potential causes of corneal eye pain, and consequently, there are many different ways patients may experience this type of symptom.
Blurred vision
Blurred vision is a common symptom of corneal ulcers. Since the cornea plays a crucial role in focusing light as it enters the eye, any disruption to its smooth surface can lead to visual distortion. The extent of blurred vision can vary depending on the size and location of the ulcer on the cornea, with larger and more centrally located ulcers causing worse visual symptoms. Patients may find that their vision fluctuates as well because the amount of discomfort and inflammation in the eye is likely to change throughout the day. The symptoms of blurry vision can often be resolved gradually after effective treatment. Other causes of blurred vision range from moderate issues, such as dry eye syndrome or refractive errors, to more severe conditions, such as glaucoma or cataracts, where the gradual clouding of the eye’s lens causes sight to become misty or hazy.
Sensitivity to Light
Approximately 20% to 25% of ulcer patients experience light sensitivity. This sensitivity may be severe or mild and can occur along with eye pain or spasms during blinking or squinting. These symptoms are compatible with erosion or abrasion of the cornea. Light sensitivity and eye pain occur most commonly when contamination or inflammatory curveballs degrade the corneal endothelium or the eye chamber (49).
Complications of Corneal Ulcers
If a corneal ulcer is not properly treated, it will continue to grow in both size and depth, becoming a longstanding ulcer. At this stage, the cornea will start to scar, which can result in permanent visual or ocular problems. The scarring of the cornea will appear gray–white and irregular, but the middle of the ulcer will clear up once the ulcer has healed. Individuals who have dense scarring in the middle layer of their cornea will have a hard time seeing through it. Light rays that enter the eye are typically refracted (bent) by the cornea, but this can’t happen if the membrane acts like a frosted window. Pieces of light will try to penetrate the corneal scar only to be reflected off it, so the only thing the person sees is a hazy, foggy failure of normal vision. If corneal ulcers recur or go untreated for a long time, they have the potential to transform into a more complex condition known as corneal perforation (50). When this happens, the infectious corneal tissue melts away, leaving a small area of weakness in the structure. Should the cornea be subject to normal pressure from the eyelids, it will likely split, allowing the aqueous humor to diffuse from within the corneal stroma. This results in the intense acute pain and edema of the cornea that characterizes this visual emergency. The emergence of a hole in the cornea results in drastic visual impairment and demands immediate and complicated therapy to prevent the loss of an eye and sight. When corneal ulcers are complicated by Endophthalmitis, which is infection of the interior of the eye, including the vitreous humor (the gel-like substance inside the eye) and sometimes the retina, it typically requires aggressive treatment, often including intravitreal injections of antibiotics and sometimes surgical intervention to remove infected tissue and prevent further spread of infection (51). If not treated, it could potentially result in severe consequences, possibly leading to the need for eye evisceration.
Diagnosis of Corneal Ulcer
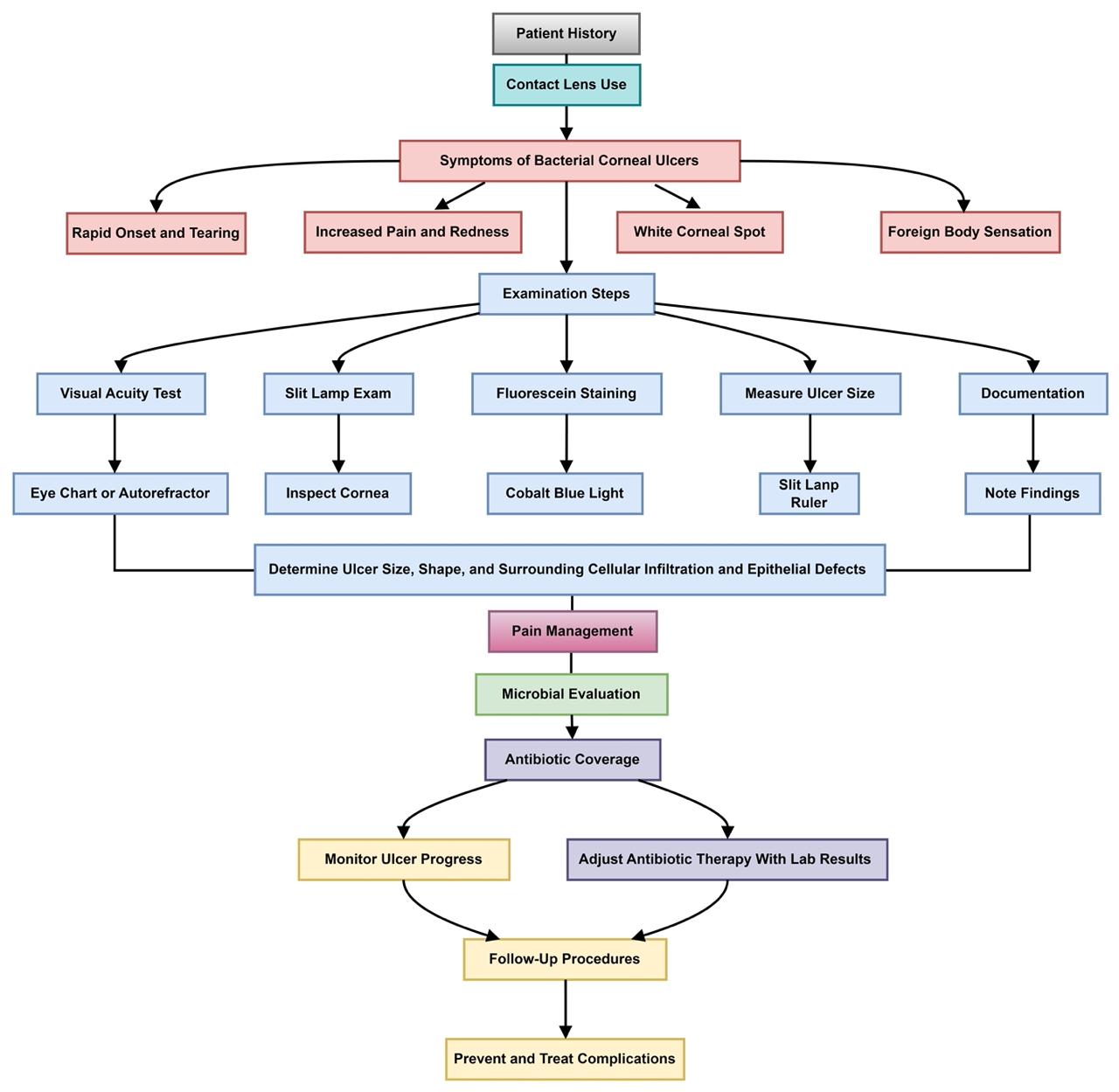
Visual examination is essential for the diagnosis and monitoring of corneal ulcers, facilitating prompt assessment and timely therapeutic intervention (Figure 1). Early detection is critical for mitigating potential complications while accurate ulcer size measurements provide a comprehensive overview of the procedural components involved in this diagnostic process (52).
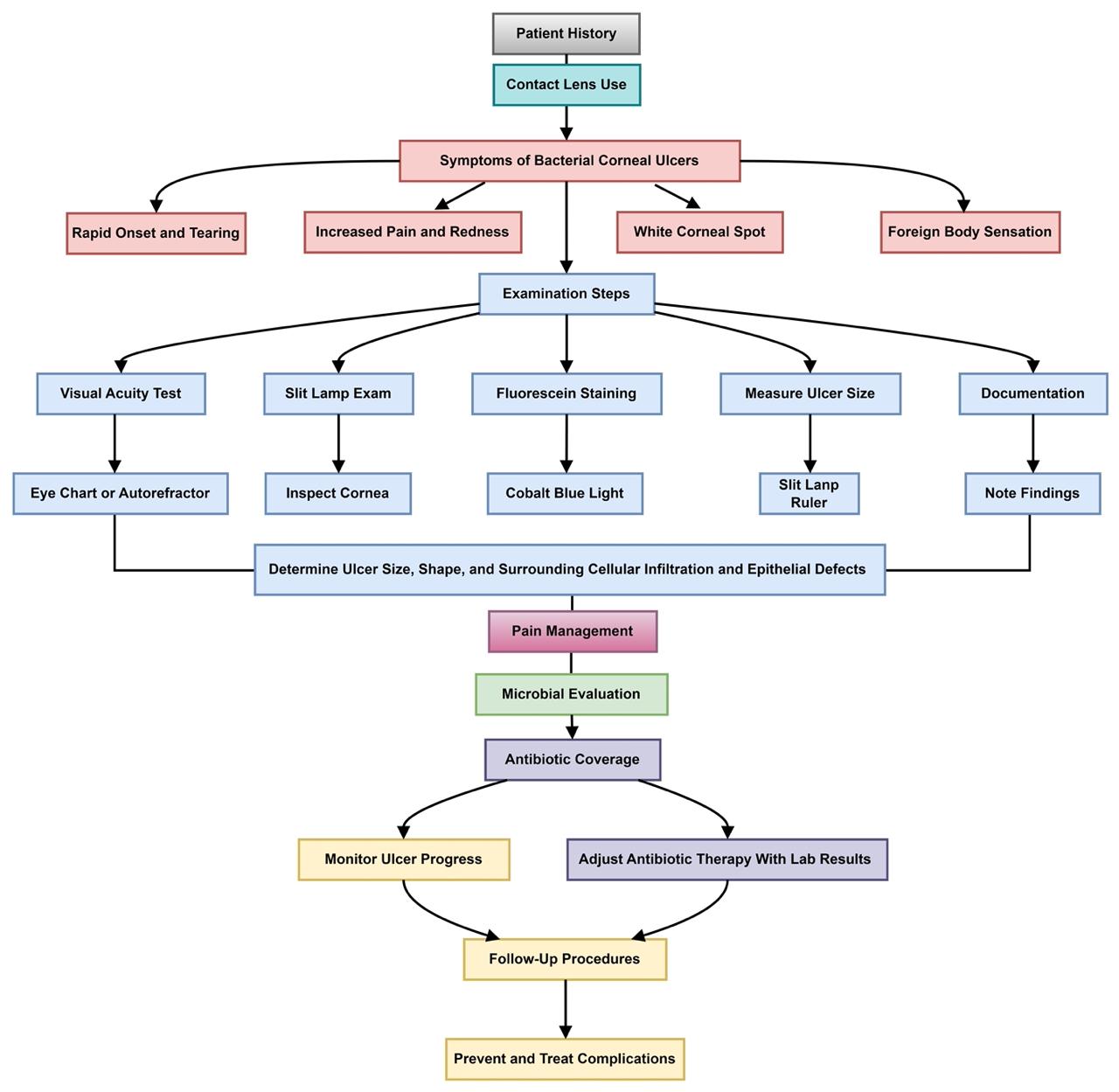
Figure 1: Comprehensive examination of Corneal Ulcer
A slit lamp examination, augmented by fluorescein dye under cobalt blue light, is routinely employed to evaluate the cornea (53). The slit lamp offers a magnified, three-dimensional visualization of the ocular surface, aiding in the detection of corneal irregularities and lesions (Figure 2). The use of fluorescein dye enhances the visualization of abrasions and ulcerative defects. To ensure patient comfort, topical anesthetic eye drops are administered prior to the application of the dye. The resultant fluorescein staining patterns and intensity variations observed during the examination enable the clinician to confirm the presence and localize the extent of corneal ulcers.
Corneal Scraping
Corneal scraping is a procedure commonly used in ophthalmology to diagnose corneal ulcers. Corneal scraping should be performed only by qualified medical professionals. This procedure involves gently removing a small sample of the tissue from the surface of the cornea using a special instrument called a kimura spatula or corneal scraper. This sample is examined under the microscope to determine the causes of any corneal abnormalities or infections. Preparation for corneal scraping is quite similar to that of most ocular surgeries. Patients will likely receive anesthetic eye drops to prevent discomfort during the procedure. It is important to ensure that the patient’s head and body are stable to prevent any injury from any sudden movement. The corneal scraping procedure commences with the cornea being scraped in a clockwise direction, initiating from the 12 o'clock position to the 6 o'clock position, followed by a reverse scraping from 6 to 12, and finally, lateral scraping from 3 to 9 o'clock. A fine needle is centered and angled laterally to utilize the sharpest portion of the blade, thereby enhancing precision during the procedure. The epithelium, the outermost layer of the cornea, is meticulously exercised using either a small blade or a rotating burr brush, a technique known as debridement. Following this, a Kimura spatula is employed to collect a sample from the corneal stroma, the middle layer of the cornea (54), which serves as the actual tissue for inoculating agar plates. In certain instances, a needle may also be utilized to aspirate fluid from the cornea, enabling the collection of deeper samples. Subsequently, the tissue sample is placed on a glass slide and dispatched to the laboratory alongside agar plates for further analysis. It is imperative that treatment begins promptly; therefore, empirical antibiotic therapy is initiated to address the bacterial infection prior to the laboratory results becoming available.
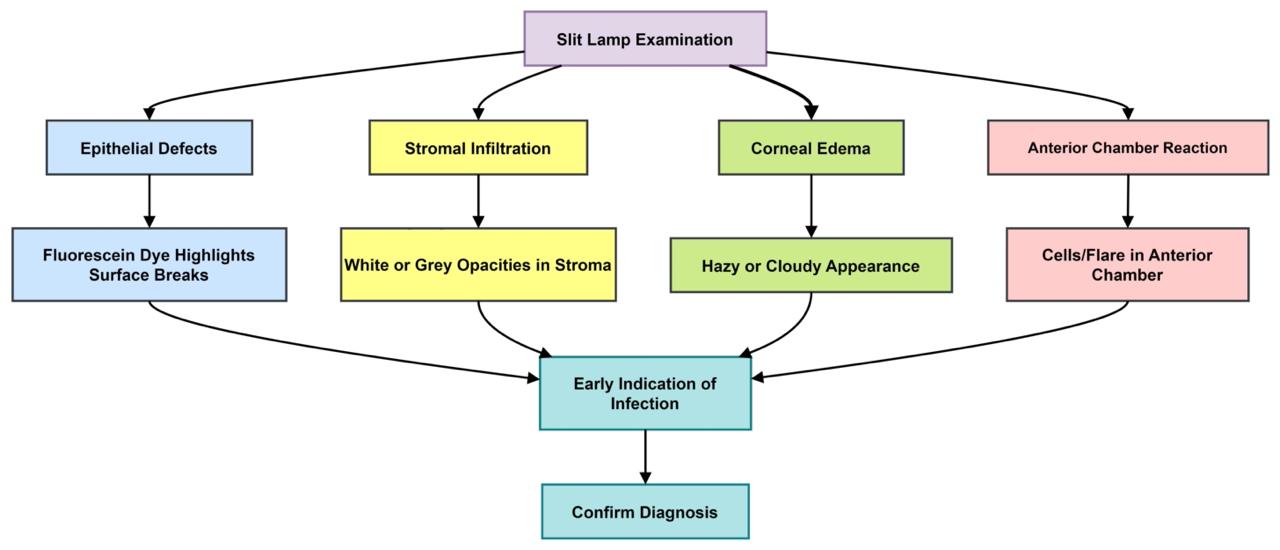
Figure 2: Corneal infection and slit lamp examination
Laboratory Techniques for Corneal Scraping
Laboratory techniques play a crucial role in detecting organisms present in eye contact lens infections (55). They also help clinicians identify drug-of-choice therapies to counter particular pathogens. Various laboratory tests and examination methods frequently show that the organisms that cause eye contact lens infections are bacteria (56). Laboratory diagnosis should start with a rapid evaluation of a fresh corneal specimen.
Staining Methods
Gram and Giemsa stains are commonly used dyes in corneal scraping microscopy. A Gram stain is a differentiation stain, which means it is used to classify bacteria. Bacteria that display a crystal violet stain even after acetone is used to dissipate the primary stain are labeled Gram positive; those decolonized with the aid of acetone will absorb the safranin counter stain and be categorized as Gram negative. Gram stains are performed by a series of staining with purple crystal violet, iodine, acetone, and safranin (57). The morphology and association of the different microorganisms within the sample can be discovered under light microscopy. This offer important insights for medical diagnosis since the results can come out within 2 hours. Giemsa staining is used in cytology. It is simple to use and can be employed rapidly, producing good results within 20 minutes. The staining is performed on a dried slide. Good results can be obtained by using freshly prepared materials. The stock solution should be filtered and stored in a dark bottle to prevent oxidation. Giemsa stain coloration involves using special dyes to color all cells of microorganisms. The microbial cells will appear purple and red due to the complex interaction between the special dyes and the proteins in the cells.
Microorganism Identification and Phenotypic Characteristics
Bacterial cultures can also be performed to identify the specific pathogens present. These cultures can be further examined using various biochemical tests to determine the exact species and strain of the pathogen inoculated based on the smear results (58). Aerobic and anaerobic bacteria cultures should be performed using appropriate solid media and other specialized media. A variety of techniques can be employed to identify bacteria and conduct antibiotic sensitivity testing (Figure 3). Automated machine systems such as VITEK, Phoenix, and MicroScan can identify the isolated organism and evaluate the sensitivity as the minimal inhibitory concentration of the antibiotic required to inhibit the growth (59-61). Manual sensitivity testing methods, such as disk diffusion, can also be used. Antibiotic disks are placed on an agar plate and incubated under optimal conditions. Zones of inhibited growth are carefully measured and compared against predetermined standards.
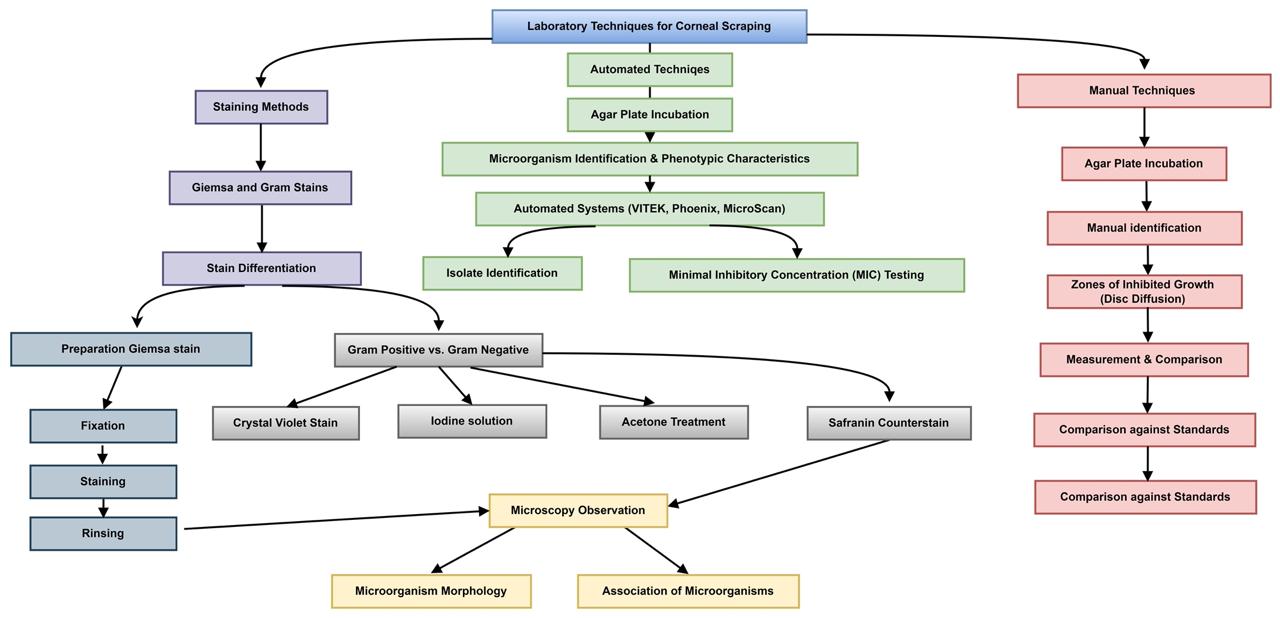
Figure 3: Laboratory procedure for Corneal Scraping
Results of culture and sensitivity tests may take up to 4 days or longer for slow-growing organisms. Preliminary results should be discussed with an ophthalmologist promptly if a specific organism is identified, such as multidrug-resistant bacteria. The results specifying the organisms’ growth and sensitivity should be filed in the patient’s medical record. Clinical progress and response to treatment should also be considered when reviewing treatment options. Aggressive or rapidly progressive infections may require escalated communication and discussion about newer or broad-spectrum antimicrobial agents
Management of Corneal Ulcers
With minimal side effects and effective treatment, antibiotics can provide therapeutic benefits to manage patients’ clinical improvement by reducing and minimizing complications and risks. A corneal ulcer is simply a loss of the corneal epithelium as the underlying stroma is infected. The rapid potential for re-epithelialization, clinical improvement, and ultimate healing outcomes suggests that antibiotics are effective at treating bacterial ulcers. Topical antibiotics are the first line of treatment for corneal ulcers (62). They achieve a high antibiotic concentration in the tear film and corneal stroma while limiting systemic concentration. Eye drops or ointments and practicing good hygiene are crucial steps in ensuring the effectiveness of treatment, preventing complications, and promoting the management of Corneal Ulcers. Moreover, Contact lenses should not be worn during infection and treatment due to the reduced effectiveness and increase risk of infection this leads to better recovery rate. Systemic antibiotics may be necessary in severe cases, especially for large or central corneal ulcers that create an inflammatory reaction or are unresponsive to initial treatments (63). The dosage should be adjusted based on the patient’s weight and organ function. The antibiotic can be placed in the lower lid pouch, with the eye closed and rolled around for an even distribution (64). Close supervision is needed to monitor adverse reactions.
Topical Antibiotics
Topical antibiotics play an important role in managing bacterial corneal ulcers by directly targeting microbial pathogens, providing broad-spectrum coverage, reducing the microbial load, and facilitating corneal healing (Figure 4). The selection of antibiotics depends on the severity of the ulcer. Initial regimens for non-severe ulcers should include coverage of the microbes that typically cause conjunctivitis such as ciprofloxacin. In cases of severe bacterial corneal ulcers where effective antibiotic concentration is needed to treat the infection effectively, fortified eye drops are typically used for cases that do not respond to standard treatments (65). They contain a higher concentration of antibiotics compared to standard eye drops. It is common for the fortified antibiotic to be mixed in the pharmacy with higher amounts of antibiotic than any available commercial
Fluoroquinolones
Exhibit concentration-dependent activity. They function by inhibiting DNA gyrase, an enzyme essential for DNA replication in bacteria and are classified as bactericidal agents. Usually, topical ciprofloxacin is applied every 1 to 2 hours at a concentration of 0.3%, whereas ofloxacin may be used less frequently at 2-hour intervals, with the provision that it also exerts a prolonged therapeutic effect after the instillation of each drop. Clinical studies have verified that fluoroquinolones are effective in reducing the time to re-epithelialization and rate of healing, with a success rate of over 70% (66).
Aminoglycosides
Are highly potent bactericidal agents that produce their effects by inhibiting protein synthesis. They are usually applied around the clock for rapid bactericidal effects. However, Aminoglycosides possess toxicity levels that have the potential to compromise the epithelium, stroma, and deep ocular tissues. Studies have shown that gentamicin is effective against Pseudomonas species but does not improve the overall primary healing time of the cornea (67). Amikacin is known to be less toxic than gentamicin but still has the potential to damage the corneal tissue.
Macrolides
Encompass a selection of antibiotic that include erythromycin, roxithromycin, clarithromycin, azithromycin, and dirythromycin. Originating from the actinomycete genus Streptomyces, macrolides antibiotics possess a macrocyclic lactone structure adjoined to multiple deoxy sugars. These compounds exert their bactericidal influence by thwarting bacterial protein synthesis (68). Accomplished through an affinity with the 50s ribosomal subunits of the bacteria, macrolides obstruct peptidyl tRNA movement, an essential step in protein assembly. Consequently, this interaction confers the antibacterial potency of the macrolides.
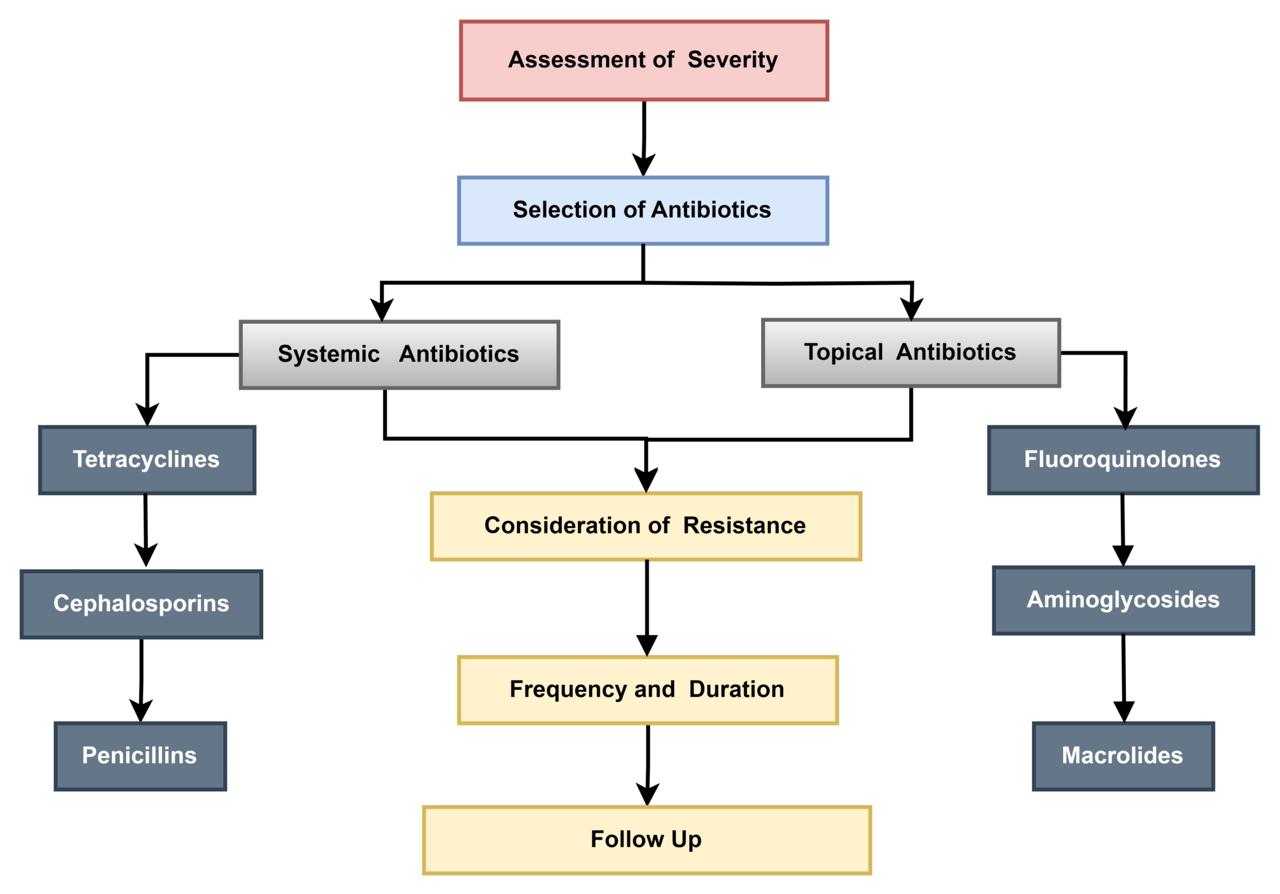
Figure 4: Antibiotic therapy as treatment approaches for corneal ulcers
Systemic Antibiotics
Systemic antibiotics refer to antibiotics that are administered orally or intravenously and are distributed throughout the body via the bloodstream. The use of systemic antibiotics can be an important addition to topical treatment, particularly with atypical bacterial keratitis in ulcers larger than 5 mm in diameter (69). This widespread distribution is what distinguishes systemic antibiotics from topical antibiotics, which are applied directly to the corneal ulcers (Figure 4).
Tetracyclines
Broad-spectrum antibiotics act to counter bacterial keratitis by penetrating the cornea. Tetracyclines exhibit an antibiotic effect on the processes of bacterial protein synthesis. The main target is the 30s ribosomal subunit of the bacterial ribosome. Tetracyclines prevent the attachment of aminoacyl-tRNA molecules to the ribosome-mRNA complex. Moreover, the anti-inflammatory properties of tetracyclines have a significant antimicrobial effect (70). An additional feature of tetracyclines is their further contribution to the effectiveness of treating bacterial keratitis. Therefore, their dual-action treatment and antimicrobial activity could make tetracyclines the drug of choice for treating bacterial keratitis. Clinical tests are continuously assessing how efficient tetracyclines in different formulations, including both topical eye drops and ointments, to establish specialized and effective treatment methods that can enhance patient prospects in bacterial keratitis cases (71).
Cephalosporins
Are second-generation synthetic β-lactam antibiotics, they inhibit the synthesis of the cell walls of bacteria, both Gram-negative and Gram-positive, through the disruption of cell wall instability. However, some bacteria are known to exert resistance to the older antibiotics of the cephalosporin class by production of β-lactamase which is an enzyme made by specific bacteria that can break down the β-lactam ring in β-lactam antibiotics. Cephalosporins are divided into several different categories, each of which has different effectiveness against bacteria. First-generation cephalosporins, such as cefazolin, are effective at controlling infections from Gram-positive bacteria by comparison, more advanced cephalosporins, such as ceftazidime, are effective at covering a deeper range of organisms, including Gram-negative aerobes (72).
Cephalosporins have a wide range of action; in addition, they have a significant advantage in terms of high tolerance and low toxicity. These drugs can be used in various categories due to its effective in the treatment of eye infections (73). The choice of a particular cephalosporin and its dosage is determined by the severity of the bacterial keratitis, the type of pathogen, and the individual characteristics of the patient. To broaden the spectrum of bacteria covered, healthcare providers may contemplate employing cephalosporins in conjunction with other antibiotics (38).
Penicillin
Derivatives, including penicillin G, penicillin V, and amoxicillin, are frequently employed in the management of conjunctivitis. Since the principal adverse effects of the use of these antibiotics in ocular disease are light due to the emergence of resistant bacterial strains. However, penicillin is effective against bacterial ocular infection but needs to be tailored to specific variants due to the differing bacterial sensitivity profiles. Penicillin G has no effect against Gram-negative bacteria and is rapidly degraded by bacterial β-lactamases; consequently, it is not advised for the management of primary bacterial keratitis (74). Another promising avenue is the use of non-antibiotic treatments. Antimicrobial peptides, for example, have broad-spectrum activity and have been shown to be effective against biofilm-forming bacteria (75-77).
Risk factors and prevention, one of the most significant contributors to bacterial keratitis and corneal ulcers in contact lens wearers, is poor hygiene. The literature consistently highlights the importance of proper lens care in preventing infections. This includes cleaning contact lenses with appropriate solutions, adhering to replacement schedules, and avoiding extended or overnight wear. Studies indicate that sleeping in contact lenses dramatically increases the risk of infection due to hypoxia oxygen deprivation in the cornea, creating a more favorable environment for bacterial growth (9). For instance, bacteria like Pseudomonas aeruginosa can grow in low-oxygen environments, making the cornea more vulnerable to infection during extended contact lens wear (78).
Despite well-established guidelines, adherence to these practices remains suboptimal among many contact lenses users. Surveys of contact lens wearers frequently report widespread noncompliance with recommended hygiene practices (10). This gap in compliance suggests that future preventive strategies should not only focus on providing education but also developing interventions that promote better adherence. This could involve technological solutions such as smart lens cases that monitor hygiene or mobile apps that remind users to replace their lenses and clean them properly.
Bacterial Colonization and Biofilm
The role of bacterial biofilm in contact lens-related infections has emerged as a critical area of research. Biofilm, which consists of communities of microorganisms embedded within a self-produced extracellular matrix, have been identified on 75% of contact lenses (78). These biofilms are particularly concerning as they enhance bacterial resistance to antibiotics, rendering infections more difficult to treat. Biofilm formation on contact lenses is a multifaceted process, involving bacterial adhesion to the lens surface, proliferation, and the synthesis of extracellular polymeric substances. Once established, these biofilms serve as reservoirs for infection, allowing bacteria to persist even after treatment. The persistence of biofilm poses a significant challenge for treatment, as many conventional antibiotics are less effective against bacteria in biofilm form (79). Consequently, the development of anti-biofilm therapies represents a critical area for future research. Nanotechnology and the use of antimicrobial peptides have shown promise in disrupting biofilm and preventing their formation on contact lenses (80). Furthermore, improving the design of contact lenses and lens care products to include anti-biofilm properties could have a significant impact in reducing the incidence of these infections (81).
Diagnosis
Speed versus accuracy. Early and accurate diagnosis of bacterial keratitis in corneal ulcers is essential for preventing complications such as scarring or perforation, which can lead to permanent vision loss. However, the diagnostic process currently relies heavily on traditional methods such as corneal scraping and microbial cultures, which, although accurate, are time-consuming. Results from microbial cultures can take several days to finalize, during which time the infection may worsen. This delay in diagnosis is particularly detrimental in rapidly progressing infections caused by aggressive pathogens like Pseudomonas aeruginosa.
The introduction of rapid diagnostic techniques, such as polymerase chain reaction PCR and point-of-care molecular testing platforms has been a promising development in this field (82). These technologies offer faster identification of bacterial pathogens and allow for more targeted antibiotic therapy, which is crucial in preventing the escalation of the infection. However, the high cost and lack of widespread availability of these technologies remain significant barriers to their routine use. Future research should focus on making rapid diagnostic methods more accessible and affordable, particularly in low-resource settings where the burden of contact lens-related infections may be higher.
Management of severe cases
In cases of severe bacterial keratitis or when treatment fails to stop the progression of the infection, surgical interventions may be required. Techniques such as corneal transplantation and the use of cyanoacrylate glue to seal small perforations have been successful in managing advanced cases (18, 83). While these procedures can restore vision and prevent the loss of the eye, they are associated with high costs, limited availability, and the risk of postoperative complications.
Recent advancements in tissue engineering and regenerative medicine hold promise for improving the management of severe corneal ulcers (84). For instance, research into the development of bioengineered corneas and stem cell-based therapies could provide new options for patients with extensive corneal damage (85). Additionally, the use of amniotic membrane grafts, which promote healing and reduce inflammation, has shown encouraging results in the treatment of non-healing ulcers. However, these treatments are still in the experimental stage and require further clinical trials before they can be widely adopted (15).
Future research directions
First, further investigation is needed into the long-term effects of prolonged low-intensity infections in contact lens wearers. These infections, though often subclinical, could have cumulative impacts on the ocular surface and overall eye health, potentially contributing to chronic inflammation, epithelial damage, or alterations in tear film dynamics.
Another crucial area for future research is the development of novel contact lens materials that are inherently resistant to bacterial biofilm colonization. Advances in material science could lead to lenses that reduce the risk of bacterial adhesion and proliferation, significantly lowering infection rates. Integrating antimicrobial agents directly into the lens material represents a promising strategy, as it would help mitigate infection risks without solely relying on user compliance with hygiene practices.
Moreover, there is a pressing need for enhanced interdisciplinary collaboration between microbiologists, ophthalmologists, and biomedical engineers to innovate more effective preventive strategies and treatment options. This cooperative approach can accelerate the development of both new materials and therapeutic interventions.
Lastly, public health campaigns aimed at educating contact lens users, particularly younger and first-time wearers, should be strengthened. By emphasizing the importance of proper hygiene practices and safe lens use, these efforts can significantly improve compliance and reduce the incidence of infections.
Future research that addresses these areas will be essential to furthering the prevention and treatment of bacterial contact lens-related infections and improving overall ocular health in contact lens wearers.
The limitations of this study include a reliance on existing literature, which may not capture the latest trends in bacterial resistance or treatment innovations. Additionally, the review focuses predominantly on bacterial pathogens, leaving out the complexities of viral, fungal, or mixed infections in contact lens wearers. There is also a lack of comprehensive data on low-resource settings, where diagnostic tools and treatment access may be limited. Furthermore, the regional variations in microbial profiles require further investigation to understand their environmental and behavioral underpinnings. Lastly, the experimental treatments mentioned, such as bioengineered corneas, remain in early stages and are not yet widely accessible.
Conclusion
The bacterial aspects of contact lenses are critical, as early diagnosis and treatment are essential to prevent complications. Innovations like point-of-care diagnostics and rapid molecular testing enhance the speed and accuracy of identification. These advancements are vital for effectively managing contact lens-related infections.
Acknowledgment
The author gratefully acknowledges Scribendi (www.scribendi.com) for the English language editing.
Disclosure
Conflict of interest
There is no conflict of interest.
Statement
No financial or personal relationship.
Funding
No funding.
Ethical Consideration
Not applicable.
Data availability
Data that supports the findings of this study are embedded within the manuscript which is based on a comprehensive literature search conducted on March 2024, in the PubMed and Web of Science.
Author Contribution
The author contributed to conceptualizing, data drafting, collection and final writing of the manuscript.