Volume 4, Issue 10
October 2024
The Genetic Overlap of Attention Deficit Hyperactivity Disorder and Autistic Spectrum Disorder: A Systematic Review
Tariq Alblawi
DOI: http://dx.doi.org/10.52533/JOHS.2024.41013
Keywords: ASD, ADHD, overlap, genetic, association, RTV
Evidence of overlap is seen in the frequent co-occurrence of attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) within individuals or families. Recently, molecular genetic techniques, have begun to identify specific single nucleotide polymorphisms and copy number variants linked to both ASD and ADHD. The objective of this systematic review is to evaluate the genetic overlap between ASD and ADHD to elucidate common genetic factors and mechanisms that contribute to both conditions. This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A comprehensive search was conducted across multiple electronic databases and studies were included if they described the genetic associations between ADHD and ASD. Screening, data extraction, and quality assessment were performed independently by two reviewers, with discrepancies resolved through discussion. The significant overlap between ADHD and ASD is evident through a complex interplay of genetic, biological, and phenotypic factors. Studies consistently reveal shared genetic influences between these disorders, with traits such as inattention and hyperactivity/impulsivity in ADHD showing moderate to strong genetic correlations with similar traits in ASD. This suggests that these characteristics may stem from common genetic factors. Notably, specific genetic markers like SHANK2 and DRD2 have been linked to both ADHD and ASD. SHANK2, in particular, appears to have a pleiotropic effect, contributing to the risk of both conditions simultaneously. Reaction time variability (RTV) also serves as a bridging factor, as it is associated with ADHD symptoms and social-communication traits in ASD. Future research should focus on expanding genetic studies to identify additional markers and clarify the mechanisms at play, exploring detailed biological pathways, conducting longitudinal studies to track symptom development, and investigating how RTV and other phenotypic traits can improve diagnosis and treatment.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are prevalent neurodevelopmental disorders that typically emerge in childhood and often persist into adulthood. Individuals with these disorders encounter two primary types of challenges: those related to general developmental changes, such as navigating new social environments and the transition to adulthood, as well as issues specific to their disorders (1). The manifestations of ADHD and ASD are varied, and their complexity is heightened by frequent comorbidities with other conditions, including gastrointestinal problems, congenital abnormalities, and immune system disorders. Both ADHD and ASD have been associated with additional psychiatric and neurological conditions, such as oppositional defiant disorder, conduct disorder, epilepsy, depression, anxiety, and substance use disorders. These disorders are also linked to significant psychosocial impairments and adverse outcomes for both patients and their families, with individuals often facing emotional and social difficulties that impact their overall quality of life (1).
ASD, characterized by difficulties in social communication and repetitive behaviours, and ADHD, marked by hyperactivity, inattention, and impulsivity, are among the most prevalent neurodevelopmental disorders, affecting almost 6%–14% of the population collectively (2). Although these conditions share several similarities, the diagnostic criteria permitting the coexistence of ASD and ADHD were only established in 2013 (2). Over the past decade, research has indicated a rising prevalence of both ADHD and ASD. Studies reveal that almost 30% to 50% of individuals with ASD exhibit ADHD symptoms, particularly in early childhood, and approximately two-thirds of those with ADHD display features of ASD (3). Recent data from the Autism Treatment Network highlights that the co-occurrence of ADHD and ASD is linked to a diminished quality of life and lower adaptive functioning compared to having either condition alone. Both disorders often involve issues with attention, peer communication, impulsivity, and varying degrees of hyperactivity. They are more frequently diagnosed in boys than in girls and are typically present by preschool age. Both disorders have a genetic predisposition, manifesting both as comorbid conditions in individuals and across family members, and they contribute to significant behavioural, academic, emotional, and adaptive challenges in various settings (3).
The relationship between ASD and ADHD has been complex. ASD is characterized by cognitive rigidity, while ADHD is marked by excessively flexible and unstable cognitive patterns. Previous diagnostic frameworks, such as DSM-IV-TR and ICD-10, did not permit the diagnosis of both conditions in a single individual. Nevertheless, research has shown that over 15% of children with ADHD display traits of ASD, and more than 40% of children with ASD exhibit characteristics of ADHD. This clinical evidence led to revisions in diagnostic criteria, allowing for the recognition of both disorders in the same person (4).
From a behavioural standpoint, particularly in diagnosis and clinical management, it can be challenging to differentiate the traits of ASD from those of ADHD. Individuals with ASD often display symptoms that overlap with ADHD, such as difficulties with attention, impulsivity, and emotional instability. Despite these similarities, the methods for behavioural and pharmacological intervention can vary significantly. A clearer understanding of the genetic relationship between ASD and ADHD could improve treatment strategies. If ASD and ADHD are manifestations of the same underlying genetic condition, a combined treatment approach might be effective for addressing both. However, if they have distinct genetic causes, separate treatments may be more effective. Therefore, a comprehensive grasp of the genetic factors contributing to the co-occurrence of ASD and ADHD is essential for refining treatment plans (5).
ADHD and ASD are both highly heritable, with estimates ranging from 60% to 93%, and their inheritance patterns are complex and polygenic. Despite these high heritability estimates, it is only recently that genome-wide association studies (GWAS) have robustly identified common genetic variants associated with each disorder. While ADHD and ASD differ in their core clinical symptoms, genetic research has revealed significant overlap between them, including a genetic correlation (rG) of 0.36 from common variations and considerable sharing of rare genetic risk variants, such as large copy number variants and protein-truncating mutations. These findings align with clinical and epidemiological evidence of overlapping phenotypic features, high comorbidity rates between the disorders in both genders and increased risk of ADHD among relatives of individuals with ASD. Identifying the genetic components that are shared or unique to each disorder could enhance our understanding of their underlying biology and help in developing targeted sub-classifications, treatment approaches, and management strategies (6). Evidence of this overlap is seen in the frequent co-occurrence of ADHD and ASD within individuals or families. Recently, molecular genetic techniques, such as GWAS, have begun to identify specific single nucleotide polymorphisms (SNPs) and copy number variants (CNVs) linked to both ASD and ADHD. The genes associated with these SNPs and CNVs may act as pleiotropic genes, contributing to the genetic overlap observed between ASD and ADHD (7).
The overlap in symptoms between ADHD and ASD is likely due to shared genetic influences affecting traits of both disorders. For instance, individuals with ADHD, as well as their siblings, exhibit more symptoms of ASD compared to controls, indicating a familial link. Twin studies have further demonstrated shared genetic factors between ADHD and ASD, with the degree of overlap increasing from 27% at age 2 to approximately 50% at age 8 and reaching 72% between ages 18 and 33 (8). Molecular genetic research has identified potential genomic regions and pathways linked to this shared heritability, although results have been inconsistent, possibly due to limited statistical power in some studies. The shared etiology of ADHD and ASD also suggests common neurocognitive pathways. However, research on these disorders has traditionally been conducted in separate domains. This separation was partly due to the fact that a dual diagnosis of ADHD and ASD was not officially recognized until the release of the DSM-5 in 2013. With increased awareness of the interplay between ADHD and ASD over the past decade, there is now a growing need to integrate and deepen the understanding of the overlapping and distinct neurocognitive processes involved in these disorders (8).
Studying the genetic overlap between ASD and ADHD is crucial from a clinical perspective because it offers insights into shared biological mechanisms that underlie both conditions. Identifying common genetic factors can enhance diagnostic accuracy, revealing why these disorders often co-occur and lead to more precise, individualized treatment plans. This genetic understanding can also drive the development of targeted therapies, improving treatment efficacy. Additionally, it aids in risk assessment and early intervention, potentially reducing the severity of symptoms. The objective of this systematic review is to evaluate the genetic overlap between ASD and ADHD to elucidate common genetic factors and mechanisms that contribute to both conditions. By systematically evaluating and synthesizing existing genetic research, this review aims to identify shared genetic risk factors and explore the biological pathways linking ASD and ADHD. The review seeks to provide a comprehensive understanding of the genetic associations between ASD and ADHD to inform clinical practices and enhance patient outcomes.
Material and Methods
The systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Definition of outcomes and inclusion criteria
This systematic review included studies describing the genetic associations between ADHD and ASD. Eligible studies discussed the possible underlying overlap mechanisms between these two prevalent disorders. Studies published between 2005 to 2024 were considered to ensure that all relevant studies are included to generate in-depth analysis. While studies were excluded if they were case reports, letters to the editor, opinion pieces, or non-peer-reviewed articles, as these sources did not provide the rigorous evidence needed for this review. Additionally, any studies that identified other neurodevelopmental or cognitive disorders were excluded to ensure that the investigation focused solely on the overlap between these two specific disorders, allowing for a detailed examination without interference from other findings.
Search Strategy
A comprehensive search across electronic databases, including PubMed, Science Direct, and Cochrane Library for publication was conducted. The search utilized a combination of specific keywords and phrases to ensure a comprehensive and targeted retrieval of relevant studies. These included terms ‘genetic’, ‘association’, ‘overlap’, ‘mechanism’, ‘autism spectrum disorder’, ‘ASD’, ‘autism’, ‘attention deficit hyperactivity disorder’, ‘ADHD’, ‘hyperactivity’. Additional sources, such as reference lists of relevant articles and reviews, were also examined to identify any additional studies that met the inclusion criteria.
Screening and Extraction
Articles with irrelevant titles were excluded from consideration. In the subsequent phase, both the full text and abstracts of the papers were meticulously reviewed to determine their compliance with the inclusion criteria. To streamline the process, titles and abstracts were organized, assessed, and scrutinized for any duplicate entries using reference management software (Endnote X8). To ensure the highest quality of selection, a dual screening approach was adopted, involving one screening for the evaluation of titles and abstracts, and another for the comprehensive examination of the entire texts. Once all relevant articles were identified, a structured extraction sheet was created to capture pertinent information aligned with the review specific objectives. Two separate researchers conducted the data extraction process independently. The gathered information included various study attributes like the author's name, publication year, country of origin, study design, and sample size.
Quality Assessment
In this systematic review, the Newcastle-Ottawa Scale (NOS) was a critical tool for assessing the quality of non-randomized studies included in the analysis that was employed. The NOS is widely recognized for its utility in evaluating the methodological quality and risk of bias in observational studies, including cohort and case-control studies. It provides a structured framework for evaluating key aspects of study design, including selection of study groups, comparability, and ascertainment of outcomes. By using the NOS, a systematical appraisal of the included studies was performed to ensure that only high-quality evidence contributed to the analysis, thereby enhancing the robustness and reliability of the review final findings.
Results
Search Results
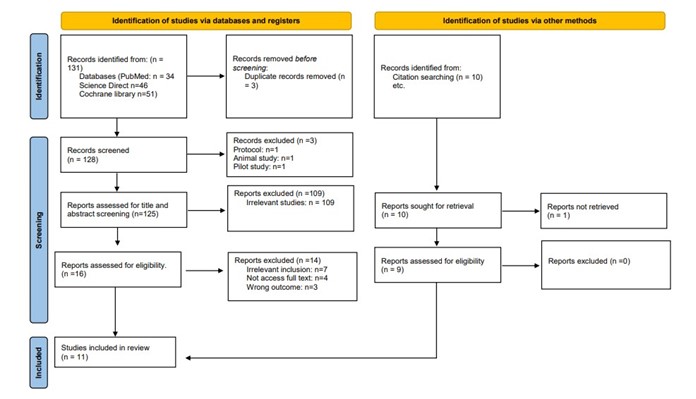
The PRISMA flow diagram illustrates the study selection process for this systematic review. Initially, 131 records were identified through database searches (PubMed, Science Direct, and Cochrane Library), with an additional 10 records found via other methods, such as citation searching. After removing 3 duplicates, 128 records were screened. Subsequently, 125 records underwent title and abstract screening, resulting in the exclusion of 109 irrelevant studies. From the remaining 16 reports assessed for eligibility, 7 were excluded due to irrelevant inclusion criteria, 4 due to inaccessible full text, and 3 for not addressing the desired outcomes. Nine studies were obtained from reference searching. Ultimately, 11 studies were included in the final review, incorporating both database and additional search methods (7, 9-18). Figure 1 provides an in-depth depiction of the search strategy and screening process.
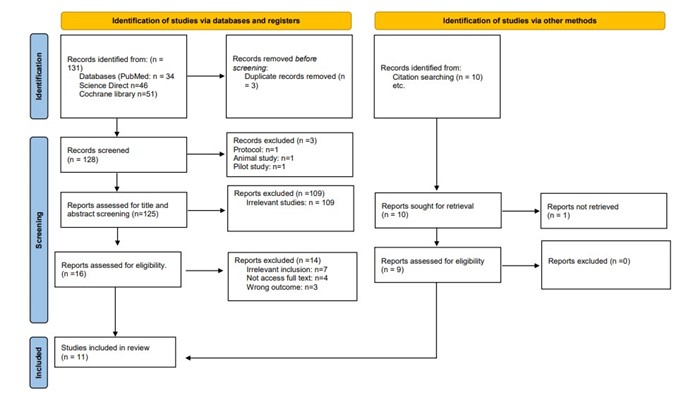
Figure 1: PRISMA Flow diagram
|
Table 1: Summary of the results of bias assessment of the included studies using the modified Newcastle-Ottawa scale (NOS) for cohort and case-control studies. |
|||||||||||
|
Author |
Year |
Selection |
Comparability |
Outcome |
Total score |
||||||
|
Representativeness of exposed cohort |
Selection of nonexposed cohort |
Ascertainment of the Exposure |
Outcome not present at the start of study |
The Subjects in Different Outcomes Groups are Comparable |
Assessment of outcome |
Length of follow-up |
Adequacy of follow-up |
||||
|
Aydin (9) |
2023 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Ronald (10) |
2008 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Pinto (11) |
2016 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Ronald (12) |
2014 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Polderman (13) |
2014 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Ghirardi (14) |
2018 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Martin (15) |
2014 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Reiersen (16) |
2008 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Naaijen (17) |
2017 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Ma (7) |
2021 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Mariggiò (18) |
2021 |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
The asterisks (*) provide a rating system ranging from 0 to 9 stars, where scores of 7 or more indicate high-quality studies, and scores less than 7 indicate low-quality studies.
Results of Quality Assessment
The quality assessment of included studies using the NOS reveals that all the studies are of high quality. These scores reflect strong adherence to criteria such as cohort representativeness, control group selection, exposure ascertainment, and adequacy of follow-up. Overall, these results suggest that the studies are generally robust (Table 1).
Characteristics of the included studies
This review encompassed 54197 participants from all included studies with a mean age range from 2 to 33.73 years. The majority of the studies were cohort-twin studies, followed by three case-control studies. Geographically, the studies varied, with three studies from the United Kingdom, two from Sweden, and each study from the United States, Australia, China, and Italy. Diverse sample sizes among the included studies were observed owing to the specific intrinsic characteristics of each study. Detailed characteristics of studies are illustrated in Table 2.
|
Table 2: Baseline characteristics of the included studies |
||||||
|
Study ID |
Study design |
Country |
Population |
Sample Size |
Male, n (%) |
Age, mean (SD) |
|
Aydin 2023 (9) |
Cohort (twin study) |
UK |
ADHD, and ASD |
566 |
271 (47.8) |
11.09 (0.74) |
|
Ronald 2008 (10) |
Cohort (twin study) |
UK |
ADHD, and ASD |
210 |
NR |
8 |
|
Pinto 2016 (11) |
Cohort (twin study) |
USA |
ADHD, and ASD |
1312 |
NR |
7-10 |
|
Ronald 2014 (12) |
Cohort (twin study) |
UK |
ADHD, and ASD |
16734 |
8534 (51) |
9-12 |
|
Polderman 2014 (13) |
Cohort (twin study) |
Sweden |
ADHD, and ASD |
17770 |
NR |
33.73 (7.6) |
|
Ghirardi 2018 (14) |
Cohort (twin study) |
Sweden |
ADHD, and ASD |
6866 |
NR |
20-28 |
|
Martin 2014 (15) |
Case-control |
UK |
ADHD, ASD, and control |
8091 |
7071 (87.4) |
10 (3) |
|
Reiersen 2008 (16) |
Cohort (twin study) |
Australia |
ADHD, and ASD |
674 |
438 (64.9) |
23 (3.75) |
|
Naaijen 2017 (17) |
Cohort |
Netherlands |
ADHD, and ASD |
946 |
828 (87.5) |
10.9 (2.8) |
|
Ma 2021 (7) |
Case-control |
China |
ADHD, ASD, and control |
773 |
773 (100) |
6-11 |
|
Mariggiò 2021 (18) |
Case-control |
Italy |
ADHD, ASD, and control |
255 |
205 (80) |
2-17 |
Study outcome measures
This review evaluated included studies to provide an in-depth analysis and explanation of the genetic overlap between ASD and ADHD (Table 3). A study by Aydin et al. examined the phenotypic and genetic relationships between theta phase variability (RTV), theta-related signals (ITC), reaction time (RTM), ADHD, and ASD. The study found a strong genetic correlation between RTV and ITC (Ra = -0.68), as well as between RTV and RTM (Ra = 0.65). Phenotypically, RTV and ITC had a moderate correlation (Rph = 0.39), while RTV and RTM showed a stronger correlation (Rph = 0.58). Using genetic multivariate liability threshold models, EEG, and cognitive tasks, the study observed that these relationships remained stable over time, highlighting a core dysregulation in the temporal coordination of control processes in ADHD. Additionally, error processing, as measured by error positivity, was disrupted in both ADHD and ASD, with significant genetic associations identified between variability in theta signaling and ADHD (9). Additionally, Ronald et al. investigated the degree of phenotypic and aetiological overlap between autistic traits and ADHD behaviors in the general population using an individual differences approach. The study revealed moderate genetic correlations (>.50), indicating notable shared genetic influences on these traits. Specifically, extreme autistic and ADHD traits had strong genetic correlations, ranging from 0.67 to 0.72. Teacher-rated traits also exhibited high heritability (0.64 to 0.72) and significant genetic overlap (rg = 0.52 to 0.59). Both autistic and ADHD traits demonstrated strong positive phenotypic correlations, with parent and teacher assessments showing correlations of 0.54 and 0.51, respectively (both p < .01). The study employed liability threshold model-fitting and cognitive ability assessments, finding that the overlap persisted even when controlling for sex, IQ, and conduct problems. Notably, 41% of children who met ASD criteria were suspected of having ADHD, and 22% of those with suspected ADHD met ASD criteria. These findings highlight the existence of common genetic influences across autistic traits and ADHD behaviors, underscoring their relevance for both research and clinical practice (10).
|
Table 3: Outcome measures of included studies |
||||||
|
Study ID |
Aim of the study |
Genetic correlation/association status |
phenotypic correlation/association status |
Methodology |
Other findings |
Conclusion |
|
Aydin 2023 |
To investigate the phenotypic and genetic relationships between theta phase variability, theta-related signals, reaction time, and ADHD and ASD |
RTV and ITC have a strong genetic correlation (Ra = -0.68). RTV and RTM also show significant genetic overlap (Ra = 0.65). |
RTV and ITC have a moderate phenotypic correlation (Rph = 0.39). RTV and RTM have a stronger phenotypic correlation (Rph = 0.58). |
Genetic multivariate liability threshold models, EEG, and cognitive tasks |
These relationships were stable across time, indicating a core dysregulation of temporal coordination of control processes in ADHD. Error processing, indexed by the error positivity, was altered in both ADHD and ASD, with a strong genetic contribution. |
Significant genetic associations found between variability in theta signaling and ADHD. |
|
Ronald 2008 (10) |
To take an individual differences approach to determine the degree of phenotypic and aetiological overlap between autistic traits and ADHD behaviours in the general population. |
Genetic correlations >.50, indicating moderate overlap in genetic influences on these traits. Strong genetic influences on extreme autistic and ADHD traits, with correlations of 0.67 to 0.72. Teacher-rated traits also had high heritability (0.64 to 0.72) and significant genetic overlap (rg = 0.52 to 0.59). |
Autistic and ADHD traits showed strong positive phenotypic correlations in the general population, with parent and teacher data at .54 and .51, respectively (both p < .01). |
Liability threshold model-fitting, and cogntive ability |
Overlap persisted even when controlling for sex, IQ, and conduct problems. Substantial overlap in suspected cases: 41% of children meeting ASD criteria had suspected ADHD. 22% with suspected ADHD met ASD criteria. |
Common genetic influences exist across autistic traits and ADHD behaviors, relevant for both research and clinical practice |
|
Pinto 2016 (11) |
To clarify the phenotypic and genetic relations between ADHD and ASD by distinguishing between symptom subscales |
Social-communication ALTs show substantial genetic overlap with ADHD symptoms, with correlations of 0.52 for inattention and 0.44 for hyperactivity-impulsivity. |
Social-communication ALTs have a moderate phenotypic correlation with ADHD symptoms (around 0.30). |
Multivariate structural equation modelling, and social communication |
RTV: Phenotypically (0.18) and genetically (0.32) associated with social-communication ALTs. Captured 24% of shared genetic influences between inattention and social-communication ALTs. |
Social-communication ALTs contribute to both phenotypic and genetic links between ADHD and ALTs. RTV, while associated with ADHD, also relates to social-communication ALTs, helping to explain the co-occurrence of ASD and ADHD. |
|
Ronald 2014 (12) |
To take a “bottom up” approach to examine the relationship between symptom domains of ASD and ADHD in childhood. |
Genetic correlations are stronger in MZ twins, with correlations of 0.46 for SIs and 0.58 for IMP, compared to DZ twins, where correlations are lower, such as 0.11 for SIs and 0.19 for IMP. This suggests a significant genetic influence on these traits |
Moderate phenotypic correlations, such as between SIs and CIs with ADHD symptoms, ranging from 0.18 to 0.53 |
Two-factor common pathway model, Principal component analysis, symptom domain analysis, and behaviour genetic analysis |
Subdomains of ASD and ADHD: Exhibit significant specific genetic and environmental influences. Highlight etiological heterogeneity within and between ASD and ADHD. Distinction between ASD and ADHD. Natural co-occurrence of specific ASD/ADHD symptom domains. More children show features of both conditions rather than complete comorbidity. |
Social-communication ALTs contribute to the genetic and phenotypic overlap between ALTs and ADHD. RTV is linked to both ADHD and social-communication ALTs, explaining part of the co-occurrence of ASD and ADHD |
|
Polderman 2014 (13) |
To examine the genetic and environmental etiology of the association between specific ASD and ADHD disorder dimensions. |
Genetic correlations with IA and HI range from 0.22 to 0.64. |
Strongest correlations with IA and HI (rp 0.33 to 0.40). |
Self-reported data on ASD dimensions social and communication difficulties, repetitive and restricted behavior and interests, and ADHD dimensions inattention, and hyperactivity/impulsivity. |
ASDr had the strongest links to IA and HI for both genders, with correlations ranging from 0.33 to 0.40. ASDsc showed moderate correlations with IA (0.29 for females and 0.35 for males) but only weak correlations with HI (0.17 for females and 0.20 for males). Sex Differences: Minimal differences observed. |
ASDr, reflecting restricted and repetitive behaviors, has the strongest links with ADHD dimensions. Genetic overlap is dimension-specific, guiding future genetic research on psychiatric comorbidity. |
|
Ghirardi 2018 (14) |
To investigate the genetic and environmental contribution to the overlap between ADHD and ASD trait dimensions in young adults. |
Highest between HI and RRB (r = 0.56). Lowest between HI and SIC (r = 0.33). |
IA correlated similarly with RRB (r = 0.33) and SIC (r = 0.32). HI had a stronger association with RRB (r = 0.38) than SIC (r = 0.24). |
IA, HI, RRB, and SIC |
Genetic and Environmental Influences: Genetic and non-shared environmental effects were similar. Shared environmental effects were minimal. |
Dimension-specific overlap between ADHD and ASD traits. |
|
Martin 2014 (15) |
To determine more broadly whether ADHD and ASD share biological underpinnings. |
There is a significant overlap in biological pathways that are enriched in both ASD and ADHD samples at various significance levels (p < .05, p < .01, p < .001). |
_ |
Pathway analyses for copy number variant. |
Biological Pathways Overlap: CNVs in ADHD and ASD affect overlapping biological pathways more than expected by chance. This overlap persists even when excluding specific CNV regions common to both disorders. |
Significant overlap of disrupted biological processes due to large rare CNVs in children with ADHD and ASD. |
|
Reiersen 2008 (16) |
To confirm the association between autistic and ADHD symptoms in a young adult twin sample assessed by self-report and investigate whether shared genetic and/or environmental factors can explain the association. |
Genetic correlation (rg) between SRS and ADHD: 0.72. |
Moderate correlation between autistic and ADHD symptoms |
Structural equation modeling using Mx software |
Best fit for SRS and ADHD included: Additive genetic effects and unique environmental effects. No sex differences. Heritability: Both ADHD and autistic traits were moderately heritable. |
A substantial proportion of genetic influences on self-reported autistic and ADHD symptoms may be shared in young adults |
|
Naaijen 2017 (17) |
To investigatee the role of glutamate and GABA genetics in ADHD severity, autism symptom severity and inhibitory performance, based on gene set analysis, an approach to investigate multiple genetic variants simultaneously. |
Glutamate gene set: Associated with HI (P = 0.009), robust after genome-wide correction. GABA gene set: Nominally associated with inhibition (P = 0.04), but not significant after multiple comparison correction. Single genes/variants: No significant associations observed. |
The analysis found a moderate correlation between ADHD symptom domains (r = 0.506, p < 0.01) and a weak correlation between hyperactivity/impulsivity and autism symptoms (r = 0.077, p < 0.05).
No significant correlations were observed between inattention and autism symptoms (r = -0.006, p = 0.86) or between clinical symptoms and SSRT (r = -0.008 to 0.094, p > 0.1). |
Genome wide association analysis |
_ |
Analysis of multiple genetic variants within gene sets revealed involvement of excitatory and inhibitory neurotransmitter systems in ADHD and ASD symptom severity in ADHD |
|
Ma 2021 (7) |
To investigate whether SHANK genes were potential pleiotropic genes to ASD, and ADHD underlying their genetic overlap. |
Allele Frequency Analysis: No significant differences in allele frequencies of SNPs in SHANK2/SHANK3 among ADHD, ASD, and ADHD + ASD groups. No significant differences between community controls and pseudo-controls. |
_ |
Cluster based algorithm for genotyping |
Aggregated Sample Comparison: Identified significant associations with ADHD and ASD for three SHANK2 SNPs: rs11236616: OR = 0.762, permuted p = 0.0376 rs7106631: OR = 0.720, permuted p = 0.0034 rs9888288: OR = 0.770, permuted p = 0.0407 Significant even after multiple testing correction. |
SHANK2 may be a pleiotropic gene contributing to the genetic overlap between ADHD and ASD, partly explaining their high comorbidity in affected children |
|
Mariggiò 2021 (18) |
To examine the DRD1 and DRD2 dopamine receptor single nucleotide polymorphisms as potential risk factors for ASD, ADHD, and ASD/ADHD overlap. |
DRD2-12 (rs7131465) shows significant associations with both ADHD (P = 0.003, corrected P = 0.015) and ASD (P = 0.005, corrected P = 0.015). DRD2-10 (rs2734832) and DRD2-8 (rs2245805) show associations but are less consistent, with DRD2-10 having P-values of 0.04 to 0.05 and DRD2-8 showing P = 0.05. DRD1-B (rs4532) and DRD1-D (rs265975) have nominal significance with controls (P = 0.04, corrected P = 0.12). |
_ |
Genotyping, and Restriction Fragment Length Polymorphism |
Potential Risk Factors: SNPs in DRD1 and DRD2 receptors might be risk factors for developing specific clinical phenotypes of ASD and ADHD. Significant Association: Only DRD2-12 (rs7131465) was significantly associated with a higher risk for the ASD/ADHD overlap. |
Data supports the hypothesis of genetic neuromodulation of the DS in the neurobiology of ASD and ADHD |
ADHD: Attention Deficit Hyperactivity Disorder; ALT: Autistic Like Traits; ASD: Autism Spectrum Disorder; ASDr: ASD Repetitive and Restricted Behavior and Interests; CI: Communication Impairments; CNV: Copy Number Variant; DS: Dopamine System; DZ: Dizygotic; EEG: Electroencephalogram; HI: Hyperactivity-Impulsivity; IA: Inattention; ITC: Intertrial Phase Coherence; MPL: Impulsivity; MZ: Monozygotic; RRB: Repetitive and Restricted Behaviors; RTM: Reaction Time Mean; RTV: Reaction Time Variability; SCI: Social Interaction and Communication; SI: Social Impairment; SNP: Single Nucleotide Polymorphism.
Moreover, Pinto et al. aimed to elucidate the phenotypic and genetic relationships between ADHD and ASD by examining symptom subscales. The study found that social-communication autistic-like traits (ALTs) exhibit substantial genetic overlap with ADHD symptoms, with correlations of 0.52 for inattention and 0.44 for hyperactivity-impulsivity. The phenotypic correlation between social-communication ALTs and ADHD symptoms was moderate, approximately 0.30. Using multivariate structural equation modeling and assessments of social communication, the study revealed that RTV was associated with both social-communication ALTs, phenotypically at 0.18 and genetically at 0.32. RTV accounted for 24% of the shared genetic influences between inattention and social-communication ALTs. This indicates that social-communication ALTs play a significant role in linking ADHD and ALTs, with RTV bridging the relationship between ADHD and social-communication ALTs, thus providing insights into the co-occurrence of ASD and ADHD (11). Another study by Ronald et al. employed a bottom-up approach to explore the relationship between symptom domains of ASD and ADHD in childhood. The study found that genetic correlations were stronger in monozygotic (MZ) twins, with correlations of 0.46 for social impairments (SIs) and 0.58 for impulsivity (IMP), compared to dizygotic (DZ) twins, where correlations were lower (0.11 for SIs and 0.19 for IMP), indicating a significant genetic influence on these traits. Phenotypic correlations between SIs and ADHD symptoms ranged from 0.18 to 0.53. Using a two-factor common pathway model, principal component analysis, symptom domain analysis, and behavioral genetic analysis, the study revealed that specific subdomains of ASD and ADHD exhibit distinct genetic and environmental influences, highlighting etiological heterogeneity within and between these disorders. It was noted that while there is a natural co-occurrence of specific ASD and ADHD symptom domains, many children display features of both conditions rather than complete comorbidity. Social-communication ALTs contribute to the genetic and phenotypic overlap between ASD and ADHD, with RTV linking both ADHD and social-communication ALTs and partially explaining the co-occurrence of ASD and ADHD (12).
While Polderman et al. investigated the genetic and environmental factors underlying the association between specific dimensions of ASD and ADHD. The study found genetic correlations with inattention (IA) and hyperactivity/impulsivity (HI) ranging from 0.22 to 0.64, with the strongest correlations between ASD dimensions and IA and HI ranging from 0.33 to 0.40. Self-reported data revealed that restricted and repetitive behaviors (ASDr) had the strongest links to IA and HI across both genders, with correlations between 0.33 and 0.40. Social and communication difficulties (ASDsc) showed moderate correlations with IA (0.29 for females and 0.35 for males) but weaker correlations with HI (0.17 for females and 0.20 for males). Minimal sex differences were observed. The study highlights that ASDr, which encompasses restricted and repetitive behaviors, has the most significant association with ADHD dimensions. The findings suggest that genetic overlap is specific to certain dimensions, providing a basis for future genetic research on psychiatric comorbidities (13). Additionally, Ghirardi et al. explored the genetic and environmental contributions to the overlap between ADHD and ASD trait dimensions in young adults. The study found that HI had the highest correlation with restricted and repetitive behaviors (RRB) (r = 0.56) and the lowest with social and interpersonal communication difficulties (SIC) (r = 0.33). Inattention (IA) showed similar correlations with RRB (r = 0.33) and SIC (r = 0.32). HI had a stronger association with RRB (r = 0.38) compared to SIC (r = 0.24). The analysis of genetic and environmental influences revealed that genetic and non-shared environmental effects were comparable, while shared environmental effects were minimal. The findings indicate that the overlap between ADHD and ASD traits is specific to certain dimensions (14).
Furthermore, Martin et al. investigated whether ADHD and ASD share common biological underpinnings. The study found significant overlap in biological pathways affected by both disorders, with pathways being enriched at various levels of significance (p < .05, p < .01, p < .001). Pathway analyses of copy number variants (CNVs) revealed that CNVs associated with ADHD and ASD impact overlapping biological pathways more frequently than would be expected by chance. This overlap remained significant even when accounting for CNV regions common to both disorders. The study highlights a substantial overlap in disrupted biological processes due to large rare CNVs in children with ADHD and ASD (15). Reiersen et al. aimed to confirm the association between autistic and ADHD symptoms in a young adult twin sample, as assessed by self-report, and to explore whether shared genetic and/or environmental factors could explain this association. The study found a high genetic correlation (rg) of 0.72 between self-reported autistic traits (SRS) and ADHD symptoms. Structural equation modeling using Mx software indicated that the best fit for the association included both additive genetic effects and unique environmental effects, with no observed sex differences. The heritability of both ADHD and autistic traits was found to be moderate. The findings suggest that a substantial proportion of genetic influences on self-reported autistic and ADHD symptoms may be shared in young adults (16).
Naaijen et al. investigated the role of glutamate and GABA genetics in ADHD severity, autism symptom severity, and inhibitory performance through gene set analysis, which examines multiple genetic variants simultaneously. The study found that the glutamate gene set was significantly associated with HI (P = 0.009), a finding that remained robust after genome-wide correction. Conversely, the GABA gene set showed a nominal association with inhibition (P = 0.04), but this was not significant after adjusting for multiple comparisons. No significant associations were observed for individual genes or variants. The analysis revealed a moderate correlation between ADHD symptom domains (r = 0.506, p < 0.01) and a weak correlation between hyperactivity/impulsivity and autism symptoms (r = 0.077, p < 0.05). However, no significant correlations were found between inattention and autism symptoms (r = -0.006, p = 0.86) or between clinical symptoms and stop signal reaction time (SSRT) (r = -0.008 to 0.094, p > 0.1). Overall, the findings suggest that excitatory and inhibitory neurotransmitter systems play a role in the severity of ADHD and ASD symptoms (17). Ma et al. investigated the potential pleiotropic role of SHANK genes in the genetic overlap between ASD and ADHD. Allele frequency analysis revealed no significant differences in SNP frequencies for SHANK2/SHANK3 among groups with ADHD, ASD, and combined ADHD + ASD, nor between community controls and pseudo-controls. However, using a cluster-based genotyping algorithm, the study identified significant associations between SHANK2 SNPs and both ADHD and ASD. Specifically, three SHANK2 SNPs showed significant results: rs11236616 (OR = 0.762, permuted p = 0.0376), rs7106631 (OR = 0.720, permuted p = 0.0034), and rs9888288 (OR = 0.770, permuted p = 0.0407), with significance maintained after correction for multiple testing. These findings suggest that SHANK2 may be a pleiotropic gene that contributes to the genetic overlap between ADHD and ASD, helping to explain their high comorbidity in affected children (7).
Mariggiò et al. investigated the role of SNPs in the DRD1 and DRD2 dopamine receptors as potential risk factors for ASD, ADHD, and their overlap. The study found that DRD2-12 (rs7131465) was significantly associated with both ADHD (P = 0.003, corrected P = 0.015) and ASD (P = 0.005, corrected P = 0.015). Although DRD2-10 (rs2734832) and DRD2-8 (rs2245805) also showed associations, these were less consistent, with DRD2-10 having P-values between 0.04 and 0.05 and DRD2-8 at P = 0.05. SNPs DRD1-B (rs4532) and DRD1-D (rs265975) showed only nominal significance (P = 0.04, corrected P = 0.12). The genotyping and restriction fragment length polymorphism analyses indicate that SNPs in the DRD1 and DRD2 receptors may be risk factors for specific clinical phenotypes of ASD and ADHD. Notably, DRD2-12 was significantly associated with an increased risk for the ASD/ADHD overlap, supporting the hypothesis of genetic neuromodulation in the neurobiology of these disorders (18). Overall, research on the overlap between ADHD and ASD highlights significant shared genetic and biological factors influencing both disorders. Studies have identified substantial genetic correlations between traits associated with ADHD and ASD.
Discussion
Findings from this systematic review reveal a complex interplay of genetic, biological, and phenotypic factors that underscore their significant overlap. The studies consistently show significant shared genetic influences between the two disorders. Traits such as inattention and hyperactivity/impulsivity in ADHD exhibit moderate to strong genetic correlations with similar traits in ASD, indicating that these characteristics may arise from common genetic factors. Specific genetic markers, including SHANK2 and DRD2, have been identified as contributing to the overlap between ADHD and ASD. SHANK2, in particular, is implicated in both disorders, suggesting that it may play a pleiotropic role, influencing the risk for both conditions simultaneously.
Shank family proteins SHANK1, SHANK2, and SHANK3 are synaptic scaffolding proteins that form a large protein complex at the postsynaptic density of excitatory glutamatergic synapses. Recent human genetic research has demonstrated that the SHANK family genes are responsible for idiopathic ASD (19). Similarly, another review by Guilmatre et al. proposed that SHANK genes may play an important role in memory and executive dysfunctions reported in a variety of neuropsychiatric diseases, including ADHD and ASD (20). Several large-scale genomic studies have suggested the relationship between cases of ASD and mutations in the genes SH3, multiple ankyrin repeat domain protein 1 SHANK1, SHANK2, and SHANK3 (21). However, the association of SHANK genes in ADHD and ASD overlap necessitates further research to draw any conclusions, as the present literature remains limited in this regard.
The DRD2 gene encodes the dopamine receptor D2, which is highly expressed in the prefrontal cortex and plays a crucial role in locomotion, reward processing, reinforcement, memory, and learning. DRD2 is a G-protein coupled receptor (GPCR) that inhibits adenylyl cyclase and is located in dopaminergic synapses. It is present in both presynaptic and postsynaptic membranes, facilitating the reuptake of dopamine into the presynaptic membrane via the DAT1 transporter. When dopamine binds to DRD2, it inhibits adenylyl cyclase, reducing intracellular cAMP levels. This change in ion concentrations across the cell membrane results in hyperpolarization, which impacts neuronal impulse transmission (22). Koevoet et al. described that numerous dopamine receptor genes have been linked to ASD. DRD1, DRD2, DRD3, DRD4, and DRD5 have all been associated with the disease. There have been reports of links between DRD3 and DRD4 polymorphisms and repetitive behaviors in ASD. This demonstrates a direct relationship between the dysfunction of dopamine and ASD symptoms (23). In comparison, results from a present case-control study indicated that after accounting for false discovery rate in terms of allele frequencies, the DRD2 gene variations rs6277 and rs6275, as well as the SLC6A3 gene variant rs2652511, were found to be strongly related with ADHD in boys and girls (24).
However, an analytical study concluded that the DRD4 7-repeat allele can increase the probability of clinically significant autistic symptoms in children and adolescents with ADHD (25). Recent neuroimaging studies have suggested that the presence of certain DRD2 and DRD4 may modify the gyrification and functional activity of cortical areas engaged in cognitive functions that are impaired in ADHD and other psychological conditions (26, 27). This cross-association of the DRD2 gene in ASD and ADHD supports the findings of this review, where it was assumed in light of the included genetic studies that DRD2 and SHANK genes may account for and explain the genetic overlap between ASD and ADHD. However, it is noteworthy here that although the available current literature supports and explains the genetic overlap of ASD and ADHD, studies defining specific genes involved or explaining this overlap on a cellular or molecular level are limited, which underscores the need for future research in this domain.
Similar to this review results, previous research has established genetic associations between ASD and ADHD; for example, twin studies in the literature show that ASD traits significantly co-occur with ADHD traits, and siblings of children with ASD diagnoses are more likely to exhibit ADHD symptoms and develop ADHD than the general population (28). In an analytical population sample's genotype data, additional evidence comes from the observation that the two diseases are genetically associated (rg = 0.36, p = 1.24E-12) (28). While twin studies of early childhood, middle childhood, and early adulthood show that some genetic factors overlap between autistic-like features and ADHD behaviours, and that the degree of overlap rises with development. Genes that influence both autistic-like and ADHD behaviours may become more effective as children grow, or new genes that influence both autistic-like and ADHD behaviours may emerge beyond age two. Children with higher ADHD behaviours demonstrated both social and non-social autistic features (29).
The pattern of relationships across relatives supports the possibility of genetic overlap between clinically diagnosed ASD and ADHD. Some characteristics of ASD are variably associated with the inattentive or hyperactive-impulsive components of ADHD (30). Similarly, studies in this review have identified distinct patterns in the genetic and environmental influences on different symptom domains. For instance, restricted and repetitive behaviors in ASD show strong associations with ADHD dimensions like inattention and hyperactivity/impulsivity, whereas social communication difficulties exhibit weaker associations. This suggests that specific subdomains of ASD and ADHD may have unique etiological pathways, though there is still considerable overlap.
Similarly, findings from a present comparative study depicted that on an individual item level, the ADHD group displayed a similar level of abnormal behaviors as the ASD group in two social-communication items: pointing and gestures. Furthermore, the frequency of stereotyped/idiosyncratic words or phrases, mannerisms, and repetitive interests and behaviors was similar among groups (31). Additionally, based on recent studies, several individuals with ADHD may suffer from social difficulties similar to those observed in ASD. The social impairments in children with ADHD are related with either relational difficulty, conduct and affective disorders, or social-communication difficulties. Children with the latter were more likely to demonstrate repetitive behaviours, speech and language difficulty, and developmental issues resembling ASD (3).
Furthermore, while analysing phenotypes the findings from this review highlight the importance of RTV as a bridging factor between ADHD and ASD. RTV, a measure of the consistency of reaction times, is associated with both ADHD symptoms and social-communication traits in ASD. This link suggests that variability in cognitive processing may be a common underlying factor connecting the two disorders. A recent study by Schachar et al. indicated that in comparison to controls, individuals with ADHD and ASD exhibited significantly longer stop-signal reaction time and RTV, with no notable differences between these two groups. ADHD traits were found to contribute to neurocognitive impairments observed in ASD, but the reverse was not true. There were no significant differences in reaction times between the groups. Similar patterns of neurocognitive deficits were identified in the community sample. However, the neurocognitive deficits in ASD were largely attributed to comorbid ADHD. Both response inhibition and sustained attention appear to be significant factors in both ADHD and ASD (32). Additionally, another study indicated that RTV is boosted in ASD only when children with comorbid ADHD are included in the sample (33).
Another study by Krakowski et al. suggests that a combined ASD-ADHD phenotype is defined by two latent ASD domains; social communication and repetitive behaviours and interests, and two latent ADHD domains; inattention and hyperactivity/impulsivity (34). Antshel and Ruso commented that ASD and ADHD share genetic inheritance and are associated with similar impairments in social and executive functioning. There are quantitative and qualitative distinctions in the phenotypic presentations of the deficits that distinguish between ASD and ADHD. Comorbid ADHD must be considered, for ASD interventions to be as effective as possible (35). Similarly, evidence from data-driven phenotypic clustering of children with ASD or ADHD showed that ASD and ADHD cannot be reliably classified as two independent clinical entities or opposite extremities of a spectrum, emphasizing the importance of studying ADHD and ASD features together (36). This explains that recent research highlights the significant overlap in phenotypic characteristics between ASD and ADHD.
Genetic aetiologies between social-communication issues and ADHD symptoms are common in the general population, and they may involve comparable biochemical pathways that co-vary over development. Population-based features are also associated with clinical disorders in both ASD and ADHD (37). In this review, one of the included studies reveals that both ADHD and ASD affect overlapping biological processes, particularly those involving neurotransmitter systems such as glutamate and dopamine. This overlap in biological pathways supports the notion that disruptions in these systems contribute to both disorders. Moreover, a study by Gadow et al. defined that the variations in dopaminergic genes may influence emotional dysregulation and ADHD symptoms in children with ASD. Specifically, a moderate association was found between the DAT1 intron8 VNTR and parent-reported emotional dysregulation (ηp2=.063). Children with the lower activity 6-6 repeat genotype exhibited more severe symptoms compared to those with the 5-6 heterozygous genotype. Notably, these associations extended to generalized anxiety, depression, and anger/irritability, and there was a moderate association (ηp2=0.110) with a composite measure of emotional dysregulation and ASD. Additionally, the DRD2 rs2283265 variant showed a connection with parent-reported emotional dysregulation (ηp2=0.041), with children homozygous for the G allele demonstrating more severe symptoms compared to heterozygotes (T-G) (38).
However, in clinical practice, it is essential to routinely screen for ADHD in children with ASD and vice versa due to their genetic overlap, which increases the likelihood of comorbidity. The previous approach of hierarchical, mutually exclusive diagnoses often led clinicians to overlook ADHD in children with ASD. This oversight is particularly detrimental because ADHD can be effectively managed with pharmacological and behavioural interventions. Without treatment, ADHD can result in considerable psychosocial difficulties for a developing child (7).
This review stands out for its inclusion of high-quality studies from a wide range of geographical locations, which enhances the robustness and generalizability of its conclusions. By synthesizing data from multiple reputable sources, the review thoroughly defines the possible genetic overlap between ASD and ADHD. Its findings are consistent with existing literature, validating previously observed trends. This alignment not only underscores the study’s reliability but also provides valuable insights into the domain, helping to refine clinical practices and improve patient outcomes. The systematic search methodology significantly enhances the strength of this study. By systematically searching for and reviewing relevant literature, the study ensures comprehensive coverage of available evidence. This rigorous approach minimizes bias, increases the reliability of results, and offers a clearer understanding of genetic overlap between ASD and ADHD.
Limitations and future research directions
This systematic review, while providing valuable insights into the overlap between ADHD and ASD, has several limitations. One major limitation is the variability in study methodologies and diagnostic criteria across the included research, which can affect the consistency and comparability of findings. Additionally, the review primarily relies on existing studies that may have sample size constraints, potentially impacting the generalizability of the results. The focus on specific genetic markers and biological pathways may also overlook other relevant factors or interactions that contribute to the disorders. Addressing these limitations in future research is crucial for gaining a more comprehensive and accurate understanding of the complex interplay between these conditions.
Future research on the overlap between ADHD and ASD will be pivotal for clinical settings by providing a more nuanced understanding of these disorders and guiding the development of targeted interventions. Identifying additional genetic markers and exploring shared neurobiological pathways will enable clinicians to better understand the underlying mechanisms of ADHD and ASD, leading to more precise diagnoses. Longitudinal studies will offer insights into how symptoms progress, allowing for earlier and more accurate identification of at-risk individuals. Advanced imaging and cognitive assessments will help in developing personalized treatment plans that address the common and unique aspects of each disorder. By integrating these findings, clinicians may implement interventions that are tailored to the specific needs of patients, potentially improving treatment efficacy and outcomes. Furthermore, future research on the overlap between ADHD and ASD should focus on several key areas to deepen the understanding of these disorders. Expanding genetic research to identify additional markers and examining their interactions with environmental factors is crucial. Longitudinal studies will help track how symptoms evolve over time, while neurobiological investigations using advanced imaging techniques can reveal shared pathophysiologies. Integrating these findings into comprehensive models of comorbidity will enhance our understanding of how the disorders intersect.
Conclusion
This systematic review underscores the significant overlap between ADHD and ASD, driven by shared genetic, biological, and phenotypic factors. Findings include the identification of common genetic markers such as SHANK2 and DRD2, which influence the risk for both disorders, and the role of RTV as a linking factor between ADHD symptoms and social-communication traits in ASD. Future research should focus on expanding genetic studies to identify additional markers and clarify the mechanisms at play, exploring detailed biological pathways, conducting longitudinal studies to track symptom development, and investigating how RTV and other phenotypic traits can improve diagnosis and treatment.
Acknowledgment
The author would like to thank King Abdulaziz University for providing the database and necessary support and guidance in performing this systematic review.
Disclosure
Statement
The author declares no conflict of interest.
Funding
None.
Ethical Consideration
Non Applicable
Data availability
All data is available within the manuscript.
Author Contribution
The author was the sole contributor to the systematic review.