Volume 4, Issue 10
October 2024
Current Trends in the Treatment and Prevention of Asthma in Children and Adults
Randa Khalil Fageeh, Mansour Mohsen Almuzaini, Robba Awad Alraddadi, Basmah Mohammed Aljohani, Zainab Zaki AL Jaffer, Eman Abdulrahman Baothman, Zahra Dheya Almajed
DOI: http://dx.doi.org/10.52533/JOHS.2024.41007
Keywords: asthma, personalized care, biologic therapies, inflammation, lifestyle modifications
Asthma, a chronic respiratory condition affecting both children and adults, remains a global health concern due to its high prevalence and significant impact on quality of life. The management of asthma has evolved significantly, with advancements in both pharmacological and non-pharmacological interventions. Traditionally, asthma treatment focused on symptom relief using inhaled corticosteroids (ICS) and bronchodilators. However, recent developments in biologic therapies, such as monoclonal antibodies targeting specific inflammatory pathways like immunoglobulin E (IgE) and interleukin-5 (IL-5), have provided new options for patients with severe, treatment-resistant asthma. The pathophysiology of asthma involves a complex interplay between genetic and environmental factors, with chronic airway inflammation playing a central role. Biomarkers such as fractional exhaled nitric oxide (FeNO) and blood eosinophil counts are increasingly used to guide treatment decisions, offering more targeted approaches for individual patients. In addition to pharmacological advancements, lifestyle modifications, including diet, physical activity and environmental control, play a crucial role in asthma prevention. A Mediterranean-style diet, rich in antioxidants and omega-3 fatty acids, along with regular exercise and reduction of allergen exposure, has been shown to reduce asthma exacerbations. Personalized asthma care is emerging as a critical trend, incorporating genetic, environmental and lifestyle factors to optimize treatment. Pharmacogenomics helps to identify patients who may respond better to specific therapies based on genetic markers. The use of digital health technologies and real-time monitoring is also contributing to more effective management by enabling timely interventions and improving patient adherence. The integration of these advancements offers a more holistic approach to asthma management, aiming to improve long-term outcomes, reduce exacerbations and enhance the quality of life for patients with this chronic condition. Future research and continued innovation will likely further refine these personalized strategies, making asthma care even more precise and effective.
Introduction
Asthma is a chronic respiratory condition characterized by inflammation and narrowing of the airways, leading to symptoms such as wheezing, shortness of breath, chest tightness, and coughing. Affecting both children and adults, asthma remains a significant global health concern, with millions of people worldwide suffering from this condition. According to the World Health Organization, approximately 262 million people were affected by asthma in 2019, with a significant number of asthma-related deaths, particularly in low- and middle-income countries (1). While asthma is a manageable disease, the burden of the condition on individuals and healthcare systems continues to be substantial.
The treatment and prevention of asthma have evolved significantly over the past few decades. Initially, asthma management was primarily focused on symptom relief through bronchodilators. However, with advancements in medical research and a better understanding of the underlying mechanisms of asthma, treatment approaches have shifted towards long-term disease control and prevention. Inhaled corticosteroids (ICS), introduced in the late 20th century, became a cornerstone of asthma management due to their effectiveness in reducing airway inflammation and preventing exacerbations (2). Moreover, recent trends highlight the importance of personalized medicine, where treatment is tailored to the individual's unique genetic, environmental, and lifestyle factors, aiming to improve disease control and reduce side effects (3). In addition to pharmacological treatments, non-pharmacological strategies, including lifestyle modifications, environmental controls and education on self-management, have become integral to asthma care. Environmental factors, such as air pollution, allergens, and exposure to tobacco smoke, play a crucial role in both the development and exacerbation of asthma, particularly in children. Preventative measures to minimize exposure to these triggers have been a focal point in asthma management and public health campaigns (4). This review aims to explore the current trends in the treatment and prevention of asthma, focusing on both pharmacological advancements and non-pharmacological strategies.
Review
Asthma management has evolved significantly over the years, with increasing emphasis on personalized approaches that consider the patient’s unique clinical profile. Traditionally, ICS has been the mainstay of long-term asthma control, effectively reducing inflammation and preventing exacerbations. However, recent advances have introduced biologic therapies that target specific inflammatory pathways, such as interleukin-5 (IL-5) and immunoglobulin E (IgE), offering new options for patients with severe asthma who do not respond to conventional treatments (5). These biologics, including omalizumab and mepolizumab, have been shown to improve asthma control and reduce hospitalizations in patients with severe, uncontrolled asthma (6).
Additionally, non-pharmacological interventions, such as lifestyle modifications and environmental control, have proven essential in asthma prevention and management. Reducing exposure to common asthma triggers, including allergens, air pollution and tobacco smoke, plays a critical role in minimizing asthma exacerbations. Moreover, patient education on self-management, adherence to treatment, and proper inhaler techniques are crucial to improving outcomes. The focus on early diagnosis and intervention, particularly in pediatric patients, is also vital to prevent disease progression and improve quality of life. Overall, the integration of pharmacological and non-pharmacological approaches has significantly improved asthma outcomes and enhanced patients' overall well-being.
Pathophysiology of Asthma
Asthma is a chronic inflammatory disease characterized by a complex interplay of genetic and environmental factors, leading to airway hyperresponsiveness, obstruction, and remodeling. The fundamental feature of asthma is chronic inflammation of the airways, which involves multiple cell types, including eosinophils, neutrophils, mast cells, and T lymphocytes, particularly the Th2 subtype. This immune response is initiated by exposure to environmental triggers such as allergens, pollutants and respiratory infections, which activate the epithelial cells lining the airways to release pro-inflammatory cytokines (7).
In allergic asthma, a key pathway involves IgE, which binds to high-affinity receptors on mast cells, leading to the release of histamine and other mediators during exposure to allergens. These mediators result in bronchoconstriction, mucus secretion and vascular permeability, contributing to the acute symptoms of asthma (8). Over time, repeated exposure to triggers and the resulting chronic inflammation led to structural changes in the airways, a process known as airway remodeling. This includes thickening of the airway wall, increased smooth muscle mass, subepithelial fibrosis, and mucus gland hyperplasia, which contributes to the fixed airway obstruction observed in severe cases of asthma (9).
Airway hyperresponsiveness, a hallmark of asthma, results from this chronic inflammation and structural remodeling. It is characterized by an exaggerated bronchoconstrictor response to various stimuli, such as allergens, exercise, cold air and respiratory irritants. In many patients, this hyperresponsiveness is due to an imbalance between pro-inflammatory and anti-inflammatory pathways. The release of cytokines such as interleukin-4, IL-5, and interleukin-13 from Th2 cells drives the recruitment and activation of eosinophils, further contributing to airway inflammation and hyperresponsiveness (7).
Non-allergic asthma, while less understood, also involves immune activation, though neutrophils may play a more prominent role than eosinophils. This phenotype is often associated with more severe, corticosteroid-resistant forms of asthma, highlighting the heterogeneity of the disease. Understanding the pathophysiology of asthma is critical for developing targeted therapies. Biologic treatments, for instance, have been developed to target specific elements of this pathway, such as monoclonal antibodies against IL-5 to reduce eosinophilic inflammation in severe asthma (9). The continuous unraveling of asthma’s complex pathophysiology holds promise for more precise and effective interventions, particularly for patients who do not respond to conventional treatments.
Advancements in Pharmacological Management of Asthma
The pharmacological management of asthma has evolved significantly over the past few decades, with a shift toward more personalized and targeted therapies. The mainstay of asthma treatment has traditionally been the use of ICS combined with long-acting beta-agonists (LABAs) to control inflammation and prevent bronchoconstriction. While these treatments are effective for many patients, recent advancements in pharmacological approaches have focused on addressing the needs of individuals with severe, treatment-resistant asthma. These advancements include biologic therapies and newer classes of medications that target specific molecular pathways involved in asthma pathophysiology.
One of the most significant advancements has been the introduction of biology, which targets key cytokines involved in asthma’s inflammatory processes. Monoclonal antibodies, such as omalizumab, which targets IgE, and mepolizumab, which targets IL-5, have been developed to treat severe asthma, particularly in patients with elevated eosinophil levels (10). These therapies have been shown to reduce exacerbations, improve lung function, and decrease the need for oral corticosteroids in patients who are inadequately controlled with conventional therapies. For example, mepolizumab has proven effective in reducing eosinophilic inflammation, a major driver of severe asthma, by blocking IL-5, which plays a central role in the recruitment and activation of eosinophils (11).
Another recent advancement in asthma management is the development of long-acting muscarinic antagonists (LAMAs) as an add-on therapy for asthma patients. Originally used to treat chronic obstructive pulmonary disease (COPD), LAMAs, such as tiotropium, have been found to be beneficial in patients with asthma who remain symptomatic despite ICS and LABA treatment. LAMAs work by blocking muscarinic receptors in the airway smooth muscle, leading to bronchodilation and reduced airway resistance. Clinical trials have demonstrated that adding tiotropium to standard therapy can improve lung function and reduce exacerbations in patients with poorly controlled asthma (12).
Further developments in asthma pharmacotherapy are focusing on novel therapeutic targets, such as the inhibition of thymic stromal lymphopoietin (TSLP), an epithelial cell-derived cytokine that plays a key role in initiating and perpetuating airway inflammation. The monoclonal antibody tezepelumab, which blocks TSLP, has shown promise in clinical trials by reducing exacerbation rates and improving lung function in patients with both eosinophilic and non-eosinophilic asthma, suggesting a broader applicability across different asthma phenotypes (13). These advancements highlight the trend toward more targeted, biologically driven treatments in asthma management, which offer new hope for patients with severe, difficult-to-control diseases. As research continues to explore novel pathways and mechanisms underlying asthma, the future of asthma treatment is likely to become even more personalized and effective.
The Role of Lifestyle Modifications in Asthma Prevention
Lifestyle modifications have emerged as an essential component in the prevention and management of asthma, offering complementary strategies alongside pharmacological interventions. These changes focus on reducing exposure to environmental triggers, improving overall health, and empowering patients with self-management techniques. A growing body of evidence highlights the importance of lifestyle factors such as diet, physical activity and environmental control in mitigating asthma symptoms and preventing exacerbations.
Diet plays a significant role in asthma prevention and management. Studies suggest that a diet rich in antioxidants, omega-3 fatty acids, and other anti-inflammatory nutrients can help reduce airway inflammation and improve lung function in asthmatic patients. The Mediterranean diet, characterized by high consumption of fruits, vegetables, fish, and olive oil, has been associated with a lower incidence of asthma symptoms, particularly in children (14). Conversely, diets high in processed foods, sugar, and unhealthy fats have been linked to increased inflammation and a higher risk of asthma exacerbations. Incorporating more nutrient-rich foods and reducing the intake of processed foods may therefore be a beneficial strategy for individuals with asthma.
In addition to dieting, regular physical activity has been shown to improve lung function and reduce asthma symptoms. Exercise improves cardiovascular fitness, enhances respiratory efficiency and reduces obesity, a known risk factor for asthma. However, certain forms of exercise, such as high-intensity aerobic activities, can induce bronchoconstriction in some individuals with asthma, known as exercise-induced bronchospasm (15). To manage this, it is important for asthmatics to engage in appropriate exercise routines, using preventive medications when necessary and ensuring proper warm-up and cool-down phases to minimize the risk of exercise-induced asthma.
Environmental control is another critical aspect of asthma prevention. Reducing exposure to common asthma triggers, such as allergens, air pollution, and tobacco smoke, has been shown to significantly lower the risk of asthma exacerbations. House dust mites, pet dander, mold, and pollen are common indoor allergens that can worsen asthma symptoms. Measures such as using allergen-proof covers, reducing humidity, and ensuring proper ventilation can help minimize exposure to these triggers (16). Moreover, avoiding second-hand smoke and reducing exposure to outdoor pollutants can further prevent asthma exacerbations, especially in urban areas where air pollution is prevalent. Overall, lifestyle modifications, when integrated with pharmacological treatments, offer a holistic approach to asthma prevention. These strategies empower patients to take control of their disease, reducing reliance on medications and improving overall quality of life.
Emerging Trends in Personalized Asthma Care
Personalized asthma care has gained significant attention in recent years as healthcare providers move toward tailoring treatments based on individual patient characteristics rather than relying on a one-size-fits-all approach. This trend is driven by the recognition that asthma is a heterogeneous disease with varying phenotypes and endotypes, which necessitates a more nuanced management strategy. Emerging trends in personalized asthma care focus on identifying specific biomarkers, optimizing treatment regimens, and incorporating genetic, environmental and lifestyle factors to improve patient outcomes.
One of the key advancements in personalized asthma care is the use of biomarkers to guide treatment decisions. Biomarkers such as fractional exhaled nitric oxide (FeNO), blood eosinophil counts, and serum IgE levels are increasingly being used to predict a patient's response to specific therapies. For instance, elevated FeNO levels have been associated with Th2-driven airway inflammation, which responds well to corticosteroid treatment (14). Similarly, patients with high eosinophil counts may benefit from biologic therapies targeting IL-5 pathways, such as mepolizumab and benralizumab. The identification of these biomarkers allows for a more targeted approach, reducing the trial-and-error process in asthma management. Genetic factors also play a significant role in asthma heterogeneity and pharmacogenomics is becoming an essential tool in personalized care. Genetic variations can influence how patients respond to asthma medications, particularly beta-agonists and corticosteroids. For example, polymorphisms in the ADRB2 gene, which encodes the beta-2 adrenergic receptor, can affect a patient’s response to beta-agonists like albuterol (17). By identifying these genetic markers, healthcare providers can optimize medication regimens, ensuring that patients receive the most effective therapies based on their genetic profile.
In addition to biological and genetic factors, environmental and lifestyle influences are being increasingly considered in personalized asthma care. Environmental exposure histories, such as exposure to allergens, air pollution, or occupational hazards, help tailor interventions aimed at minimizing these triggers. Furthermore, digital health technologies, including wearable devices and mobile health apps, are providing patients and clinicians with real-time data on symptoms, medication use, and environmental conditions. These technologies support personalized management by enabling timely interventions and fostering better patient adherence to treatment plans (18). The future of asthma care is likely to see an increasing reliance on personalized approaches, with therapies becoming more tailored to individual patient profiles. This shift toward precision medicine holds great promise for improving asthma control, reducing exacerbations, and enhancing the quality of life for patients with this complex disease.
Hyperbaric Oxygen Therapy in Asthma
Hyperbaric oxygen therapy (HBOT) involves the administration of pure oxygen at pressures higher than atmospheric levels and has been explored as a potential treatment for a variety of conditions, including asthma. The rationale behind using HBOT for asthma is based on its ability to reduce inflammation, promote tissue repair, and enhance oxygen delivery to the lungs. In asthma, where chronic airway inflammation and bronchoconstriction limit oxygen exchange, HBOT has been investigated as a supplementary therapy to improve respiratory function and reduce symptoms.
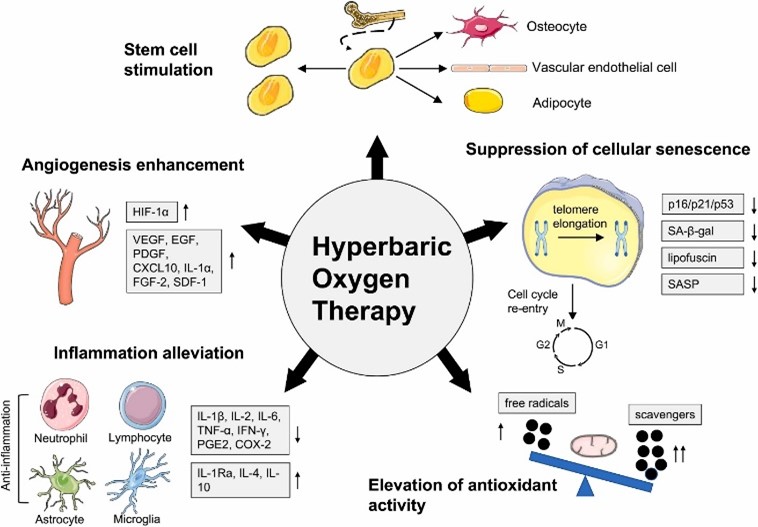
Studies have shown that HBOT may have anti-inflammatory effects by reducing the levels of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (19). These cytokines are typically elevated in asthmatic patients, contributing to airway inflammation and hyperresponsiveness. By reducing inflammation, HBOT could potentially alleviate the chronic inflammation associated with asthma, leading to improved lung function and a decrease in exacerbation frequency. Although promising, the research on HBOT in asthma is still in its early stages, and more clinical trials are needed to confirm these benefits (Figure 1).
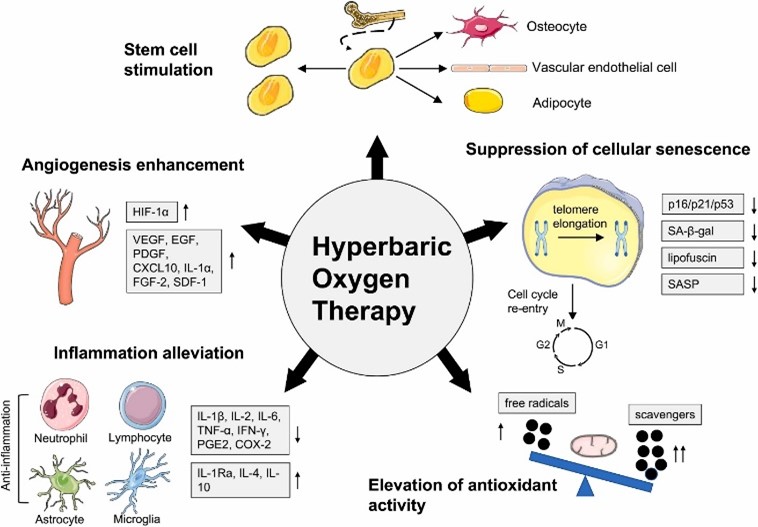
Figure 1: HBOT reduces inflammation by regulating the number and activity of extensive inflammatory cell types (20).
HBOT may also improve asthma outcomes by enhancing oxygen delivery to the alveoli, thereby improving oxygen saturation levels in patients experiencing acute asthma exacerbations. In severe cases of asthma where hypoxemia is present, oxygen supplementation is critical. HBOT offers a higher concentration of oxygen than conventional methods, which can be particularly beneficial in cases of life-threatening asthma attacks or status asthmaticus. Some small-scale studies have suggested that HBOT may improve arterial oxygenation in asthmatic patients during acute exacerbations, although the long-term benefits remain unclear (21).
Despite the potential benefits, the use of HBOT in asthma is not without risks. Hyperbaric therapy can lead to oxygen toxicity, particularly with prolonged exposure, which may exacerbate respiratory symptoms or cause other complications such as barotrauma (22). Additionally, the accessibility and cost of HBOT limit its widespread use, making it a less practical option for routine asthma management. Given the current evidence, HBOT is considered an experimental treatment for asthma and should only be used in a controlled clinical setting.
Conclusion
Advancements in both pharmacological and non-pharmacological approaches have significantly improved asthma management, enabling more personalized and effective care. The integration of biomarkers, genetic profiling, and lifestyle modifications is paving the way for tailored treatment strategies that address individual patient needs. As research continues, personalized asthma care is likely to further enhance outcomes and quality of life for patients across all age groups.
Disclosures
Author Contributions
The author has reviewed the final version to be published and agreed to be accountable for all aspects of the work.
Ethics Statement
Not applicable
Consent for publications
Not applicable
Data Availability
All data is provided within the manuscript.
Conflict of interest
The author declares no competing interest.
Funding
The author has declared that no financial support was received from any organization for the submitted work.