Volume 4, Issue 9
September 2024
Tirzepatide Safety and Efficacy in Managing Type 2 Diabetes and Obesity: A Systemic Review and Meta-Analysis
Abrar Abdullateef Soqati
DOI: http://dx.doi.org/10.52533/JOHS.2024.40902
Keywords: Tirzepatide, type 2 diabetes mellitus, obesity, management, glycemic control, weight loss
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that has shown potential in managing type 2 diabetes mellitus (T2DM) and obesity. This systematic review and meta-analysis aim to evaluate the efficacy and safety of tirzepatide by consolidating evidence from relevant studies. A systematic search of electronic databases such as PubMed, Scopus, Cochrane Library, Web of Science, and ScienceDirect was done to identify randomized controlled trials (RCTs) and clinical trials evaluating the efficacy and safety of tirzepatide in adult participants with T2DM and/or obesity. The inclusion criteria required studies to report results on glycemic control (HbA1c levels), weight loss, and adverse events. Two researchers extracted data separately. The Cochrane Risk of Bias tool was used to assess methodological quality and potential bias. Statistical analyses were conducted using R software 4.2.2. Heterogeneity among studies was assessed using the Cochrane Q p-value and I² statistic, with publication bias evaluated through funnel plots and Egger's regression test. The present study included 6 articles from 2018 to 2023. The quality assessment revealed some methodological concerns, but most studies demonstrated a low risk of bias in key areas. Tirzepatide led to a significant reduction in HbA1c levels (MD: -2.5078; 95% CI: [-3.3945, -1.6210]), fasting blood glucose (FBG) (MD: -62.78; 95% CI: [-67.9269, -57.6337]), waist circumference (MD: -8.60; 95% CI: [-15.5491, -1.6410]), and body weight (MD: -10.7852; 95% CI: [-14.8264, -6.7439]) compared to placebo. A non-significant trend towards fewer serious adverse events (RR: 0.7423; 95% CI: [0.4771, 1.1547]) and reduced mortality risk (RR: 0.2727; 95% CI: [0.0286, 2.2766]) was observed. Tirzepatide appears promising in managing type 2 diabetes and obesity, significantly enhancing glycemic control, reducing body weight, and decreasing waist circumference, all while maintaining a favorable safety profile. Further research is warranted to address methodological concerns and confirm long-term benefits and risks.
Introduction
Type 2 diabetes is chronic condition that happens when you have persistently high blood sugar levels (hyperglycemia) and is recognized as a serious public health concern worldwide. It has a considerable impact on human life and health expenditures. Obesity is a well-established modifiable risk factor for developing type 2 diabetes mellitus (T2DM), creating a complex interplay between the two conditions that complicates diabetes management and exacerbates associated health issues. Alarmingly, there have been consistent increases in the prevalence of overweight and obesity due to the rapid development of global urbanization, and modernization has lasting effects on lifestyle aspects such as unhealthy eating habits, a lack of exercise, increased stress, and environmental factors. These factors contribute to the alarming growth of obesity and type 2 DM worldwide. According to the WHO, obesity accounts for 44% of diabetes, and the incidence of obesity-related diabetes is expected to double to 300 million by 2025 (1).
Obesity and T2DM are linked to a multitude of metabolic complications, including hypertension, elevated cardiovascular risk, obstructive sleep apnea, dyslipidemia, and non-alcoholic fatty liver disease (2). Given the strong association between obesity and T2DM, weight loss in individuals with T2DM can lead to substantial enhancements in glycemic control, insulin sensitivity, and various cardiovascular parameters (3). Traditional antidiabetic drugs are primarily concerned with glucose control, but they often fail to address weight management. In contrast, weight-loss medications may not effectively regulate blood glucose levels for those with T2DM. This therapeutic gap highlights the need for novel medications that can successfully manage hyperglycemia and obesity, improving overall metabolic health and minimizing the risk of challenges.
In recent years, Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have emerged as promising medications for managing T2DM, offering effective glycemic reductions alongside clinically significant weight loss and maintenance (4). Notably, GLP-1 receptor agonists like liraglutide and semaglutide have demonstrated efficacy in glycemic control and weight management, setting the stage for exploring the therapeutic potential of dual incretin agonism (5). Combining the other incretin hormone, glucose-dependent insulinotropic polypeptide (GIP), despite its similarity with GLP-1 and its receptors, does not inhibit appetite and food intake as GLP-1 does. However, GLP-1 has shown robust weight loss effects independent of insulin sensitivity and lipid metabolism. Therefore, it has been hypothesized that combining GLP-1 agonists with agents that act on GIP receptors could produce more effective glycemic control and significant weight reduction (6). Clinical evidence indicates that this combined approach results in superior improvements in glycemic control and body weight compared to selective GLP-1 receptor agonists alone.
Tirzepatide, a novel dual GIP and GLP-1 receptor agonist, has emerged as a promising therapeutic candidate for managing T2DM and obesity. Tirzepatide has a special mode of action aiming to enhance glycemic management and encourage notable weight loss by utilizing the combined actions of these secretion hormones. The molecule is administered as a weekly subcutaneous injection (7). Preliminary clinical trials have shown encouraging results, indicating its potential to revolutionize the treatment landscape for patients suffering from these conditions. This innovative approach addresses the complexities of T2DM and obesity, providing a multifaceted solution to these widespread health challenges (8, 9).
In consideration of emerging information from clinical studies exploring the effect of tirzepatide on glycemic control and other added benefits, more thorough data is required to synthesize the findings and guide future research and potential clinical application. As a result, the purpose of this study is to give a comprehensive review and meta-analysis of the literature published on tirzepatide, with a special emphasis on the efficacy and safety of tirzepatide in the management of T2DM and obesity.
Material and methods
Definition of outcomes with inclusion and exclusion criteria
The analysis includes studies evaluating the effectiveness and safety of Tirzepatide in managing type 2 diabetes and/or obesity. It specifically focuses on randomized controlled trials and clinical trials involving adult participants diagnosed with T2DM and/or obesity. The selected studies must report outcomes related to glycemic control such as HbA1c levels, weight loss, and adverse events. Additionally, inclusion is limited to studies published in the English language with full-text availability. Studies not centered on Tirzepatide as an intervention are excluded from consideration, as are animal studies, review articles, commentaries, and editorials. Furthermore, studies exclusively involving participants with type 1 diabetes, those lacking sufficient data or complete outcomes, and studies published in languages other than English are excluded. Additionally, studies without full-text availability are excluded from the analysis.
Search Strategy
We conducted a comprehensive search across electronic databases, including Scopus, PubMed, Cochrane Library, Web of Science, and ScienceDirect for publication. The search strategy employed terms: ("Type 2 diabetes OR Diabetes mellitus, type 2 OR Obesity OR Overweight OR Insulin resistance OR Metabolic syndrome OR Hyperglycemia OR Adiposity) AND (Tirzepatide OR LY3298176 OR Dual GIP and GLP-1 receptor agonist OR Novel diabetes medication OR GLP-1 agonist OR GIP receptor agonist) AND (Placebo OR Standard care OR Comparative treatment OR Active comparator) AND (Glycemic control OR HbA1c reduction OR Weight loss OR Body mass index OR Cardiovascular outcomes OR Adverse events OR Safety OR Hypoglycemia OR Gastrointestinal side effects) focuses on identifying relevant studies assessing the efficacy and safety of Tirzepatide compared to placebo, standard care, or other comparative treatments in managing type 2 diabetes and/or obesity. The outcome measures considered include glycemic control (evaluated via HbA1c levels), weight loss (determined by changes in body mass index (BMI)), and adverse events. This comprehensive approach aims to gather evidence on the therapeutic effects and safety profile of Tirzepatide in these populations, providing valuable insights for clinical practice and further research.
Screening and extraction
Articles with irrelevant titles were excluded from consideration. In the subsequent phase, both the full text and abstracts of papers were meticulously reviewed to determine their compliance with the inclusion criteria. To streamline the process, titles and abstracts were organized, assessed, and scrutinized for any duplicate entries using reference management software (Endnote X8). To ensure the highest quality of selection, a dual screening approach was adopted, involving one screening for the evaluation of titles and abstracts, and another for the comprehensive examination of the entire texts. Once all relevant articles were identified, a structured extraction sheet was created to capture pertinent information aligned with our specific objectives.
Two separate researchers conducted the data extraction process independently. The gathered information included various study attributes like the author's name, publication year, country of origin, study design, sample size, duration of follow-up, and sources of funding. Additionally, details regarding participants, such as age, gender, and nationality, were also collected. It aims to comprehensively evaluate key outcomes including glycemic control, weight management, and adverse events. By synthesizing data from relevant studies, this analysis seeks to provide a thorough understanding of tirzepatide's impact on these parameters, offering valuable insights into its therapeutic potential and safety profile for individuals with T2DM and obesity.
Quality Assessment
We employed the Cochrane Risk of Bias assessment tool to evaluate the methodological quality and risk of bias in randomized controlled trials included in this review.
Data Synthesis
Statistical analyses were conducted using R software 4.2.2. For binary variables, and risk ratios (RR) with 95% confidence intervals (CI) were calculated. Continuous outcomes were analyzed using the mean differences [SE] with a 95% CI. Heterogeneity among studies was assessed using the Cochrane Q p-value and I² statistic, with a random-effects model and common effect model. Publication bias was evaluated using funnel plots and Egger's regression test.
Results
Search Results
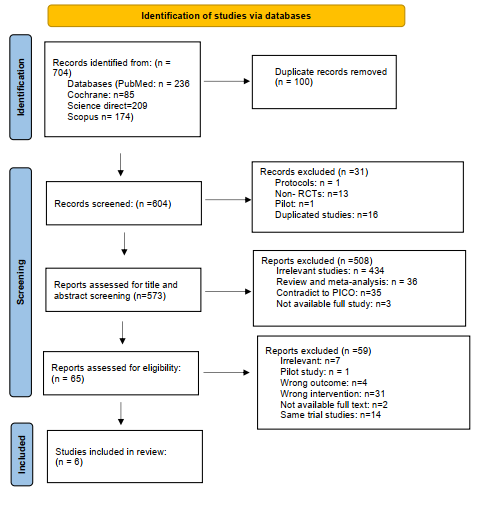
We executed the search methodologies outlined previously, resulting in the identification of a total of 704 citations, subsequently reduced to 604 following the removal of duplicates. Upon screening titles and abstracts, only 65 citations met the eligibility criteria for further consideration. Through full-text screening, this number was further refined to 6 articles aligning with our inclusion and exclusion criteria (10-15). Figure 1 provides an in-depth depiction of the search strategy and screening process.
Results of Quality Assessment
The assessment of several studies reveals a mixed picture regarding their methodological rigor and risk of bias across various domains. While most studies demonstrate commendable practices in blinding of participants and personnel, selective reporting, and absence of other biases, there are notable areas of concern. The lack of clarity or uncertainty in random sequence generation, allocation concealment, blinding of outcome assessment, and incomplete outcome data in certain studies raises questions about the reliability and validity of their findings. Nonetheless, the majority of studies present robust evidence with low risk of bias, suggesting that the reported results are generally trustworthy. Moving forward, addressing these methodological shortcomings and ensuring transparent reporting practices will be crucial for enhancing the quality and credibility of future research in the field (Table 1 and Figure 2).
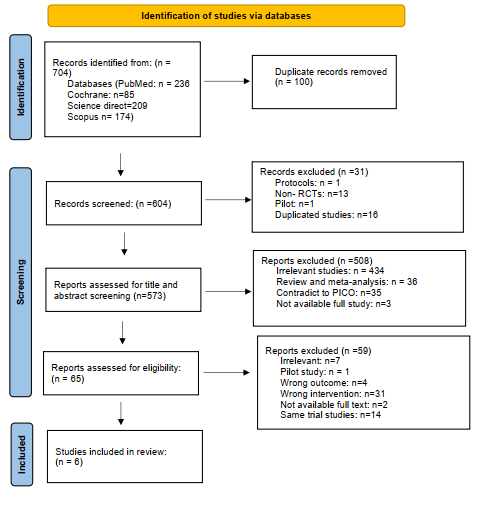
Figure 1: PRISMA flow chart of included studies
Characteristics of the included studies
Table 2 summarizes clinical trials conducted by various authors, all funded by Eli Lilly and Company, across multiple countries. The studies, spanning from 2018 to 2023, are randomized controlled trials with varying treatment durations from 12 to 72 weeks. Participants' ages ranged from mid-40s to early 60s, with gender distributions reported in each trial. Jastreboff et al. (10), involved multiple countries and had a 72-week duration with over 600 participants in each group. Frias et al. (11, 12) conducted two studies in 2018 and 2020, one solely in the USA with a 12-week period, and another across four countries with a 26-week duration. Rosenstock et al. (13) and Heise et al. (14) reported their studies with treatment durations of 40 and 28 weeks respectively, across multiple countries. Pirro et al. (15) also conducted a 26-week trial with a smaller sample size.
|
Table 1: Cochrane Risk of Bias tool for RCTs |
|||||||
|
Study |
Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective reporting |
Other bias |
|
Jastreboff et al., 2022 (10) |
Low |
Unclear |
Low |
Unclear |
Low |
Low |
Low |
|
Frias et al., 2020 (11) |
Unclear |
Unclear |
Low |
Unclear |
Low |
Low |
Low |
|
Frias et al., 2018 (12) |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Rosenstock et al., 2021 (13) |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Heise et al., 2023 (14) |
Unclear |
Unclear |
Low |
Unclear |
Unclear |
Low |
Low |
|
Pirro et al., 2022 (15) |
Unclear |
Unclear |
Low |
Unclear |
Low |
Low |
Low |
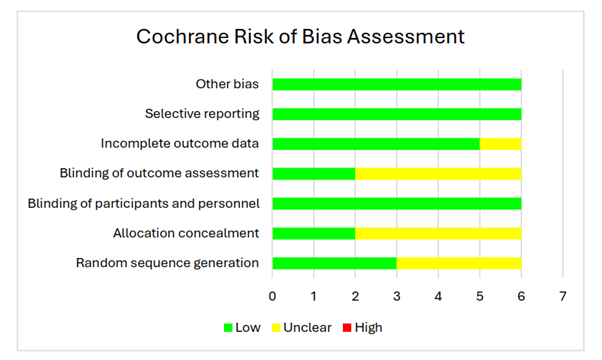
Figure 2: The Cochrane risk of bias assessment
|
Table 2: Baseline characteristics of participants in included studies |
|||||||||||||
|
Author |
Trial registration |
Country |
Year |
Study design |
Study period |
Treatment duration (weeks) |
Study participants |
Mean age |
Gender(M/F) |
Funding |
|||
|
Intervention |
control |
Intervention |
control |
Intervention |
control |
||||||||
|
Jastreboff et al. 2022 (10) |
NCT04184622 |
Argentina, Brazil, China, India, Japan, Mexico, Russian federation, Taiwan and US |
2022 |
RCT |
December 2019 to April 2022 |
72 |
630 |
643 |
44.9±12.3 |
44.4±12.5 |
32.5/67.5% |
32.2/67.8% |
Eli Lilly |
|
Frias et al. 2020 (11) |
NCT03311724 |
USA |
2020 |
RCT |
November 2017 to April 24, 2018 |
12 |
28 |
26 |
56.6 ± 9.21 |
56.0 ± 10.13 |
82.1/17.9% |
46.2 /53.8% |
Eli Lilly and Company, Indianapolis |
|
Frias et al. 2018 (12) |
NCT03131687 |
Poland, Puerto Rico, Slovakia, and USA |
2018 |
RCT |
May 24, 2017, and March 28, 2018 |
26 |
53 |
51 |
56?0 ±7?6 |
56?6 ± 8?9 |
41/59% |
57% /43% |
Eli Lilly and Company |
|
Rosenstock et al. 2021 (13) |
NCT03954834 |
India, Japan, Mexico, and the USA |
2021 |
RCT |
June 3, 2019, to Oct 28, 2020, |
40 |
121 |
115 |
52?9 ±12?3 |
53?6 ±12?8 |
52/48% |
49/51% |
Eli Lilly and Company |
|
Heise et al. 2023 (14) |
NCT03951753 |
NR |
2023 |
RCT |
NR |
28 |
45 |
28 |
61.1 ± 7.1 |
60.4 ± 7.6 |
68.9/ 31.1% |
75/ 25% |
Eli Lilly and Company |
|
Pirro et al. 2024 (15) |
NCT03131687 |
NR |
2022 |
RCT |
NR |
26 |
40 |
44 |
55.0 ±7.8 |
56.5±9.1 |
35/65% |
50/50% |
Eli Lilly and Company |
RCT: Randomized Control Trial, NR: Not Reported
Main Findings of Included Studies
Jastreboff et al. (10), demonstrated significant weight loss with tirzepatide across various dosages compared to placebo, with improvements in cardiometabolic measures and manageable adverse events. Frias et al. (11, 12) and Rosenstock et al. (13), further confirmed the benefits of tirzepatide in reducing HbA1c, fasting glucose, and achieving treatment targets, with favorable outcomes in weight management and minimal adverse events. Heise et al. (14) and Pirro et al. (15), highlighted additional benefits such as appetite reduction and modulation of metabolites associated with insulin resistance and future diabetes risk. Overall, tirzepatide emerges as a promising therapeutic option for addressing the complex interplay between type 2 diabetes and obesity, offering substantial improvements in glycemic control, weight management, and metabolic health, supported by a favorable safety profile (Table 3).
Outcomes of the Meta-analysis
Tirzepatide 15 mg compared to placebo on HbA1c level
The meta-analysis aimed to compare the mean difference (MD) in HbA1c levels between individuals treated with Tirzepatide 15 mg and those administered a placebo across three studies. The results indicated substantial reductions in HbA1c levels with Tirzepatide treatment compared to placebo.
Pooling the results under both the common effect model and random effects model revealed significant reductions in HbA1c levels with Tirzepatide treatment compared to placebo. The common effect model yielded an MD of -2.7715 with a 95% confidence CI of [-2.8560; -2.6871], while the random effects model yielded an MD of -2.5078 with a wider 95% CI of [-3.3945; -1.6210]. Both models exhibited statistically significant results, with p-values < 0.0001, indicating strong evidence of the efficacy of Tirzepatide in reducing HbA1c levels.
|
Table 3: Main findings of included studies |
|
|
Study |
Main Findings |
|
Jastreboff et al., 2022 (10) |
Initially, participants had a mean body weight of 104.8 kg and a mean BMI of 38.0, with 94.5% having a BMI of 30 or higher. Over 72 weeks, the mean percentage weight loss was -15.0% (95% CI, -15.9 to -14.2) with 5 mg doses of tirzepatide, -19.5% (95% CI, -20.4 to -18.5) with 10 mg doses, and -20.9% (95% CI, -21.8 to -19.9) with 15 mg doses, compared to -3.1% (95% CI, -4.3 to -1.9) with placebo (P<0.001 for all comparisons with placebo). The proportion of participants achieving a weight reduction of 5% or more was 85% (95% CI, 82 to 89) with 5 mg, 89% (95% CI, 86 to 92) with 10 mg, and 91% (95% CI, 88 to 94) with 15 mg of tirzepatide, contrasting with 35% (95% CI, 30 to 39) with placebo. Additionally, 50% (95% CI, 46 to 54) and 57% (95% CI, 53 to 61) of participants in the 10 mg and 15 mg groups respectively achieved a reduction in body weight of 20% or more, compared to 3% (95% CI, 1 to 5) in the placebo group (P<0.001 for all comparisons with placebo). Tirzepatide demonstrated improvements in all prespecified cardiometabolic measures. The most common adverse events were gastrointestinal, mostly mild to moderate, occurring mainly during dose escalation. Adverse events led to treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5 mg, 10 mg, and 15 mg tirzepatide doses, and placebo, respectively. |
|
Frias et al., 2020 (11) |
A total of 111 patients were enrolled in the study, distributed across the following groups: placebo (26 patients), tirzepatide 12 mg (29 patients), tirzepatide 15 mg-1 (28 patients), and tirzepatide 15 mg-2 (28 patients). The average age of participants was 57.4 years, with an HbA1c level of 8.4% and a body mass index of 31.9 kg/m2. By week 12, there was a notable difference in absolute HbA1c change from baseline (SE) between the tirzepatide treatment groups and the placebo group (placebo: +0.2% [0.21]; 12 mg: −1.7% [0.19]; 15 mg-1: −2.0% [0.20]; 15 mg-2: −1.8% [0.19]). The incidence of nausea varied across groups: placebo (7.7%), 12 mg group (24.1%), 15 mg-1 group (39.3%), and 15 mg-2 group (35.7%). Adverse events led to treatment discontinuation in three patients, one from each of the placebo, 12 mg, and 15 mg-1 groups. |
|
Frias et al., 2018 (12) |
From May 24, 2017, to March 28, 2018, 555 participants were screened for eligibility, with 318 ultimately randomized into one of six treatment groups. Due to two participants not receiving treatment, the modified intention-to-treat and safety populations comprised 316 participants. Over the course of the study, 258 participants (81.7%) completed 26 weeks of treatment, while 283 (89.6%) completed the entire study period. At baseline, the mean age was 57 years, with a mean BMI of 32.6 kg/m² and HbA1c level of 8.1%. Most participants were men (53%), and 47% were women. At 26 weeks, the effect of LY3298176 on HbA1c change was dose-dependent and continued to improve without plateauing. LY3298176 demonstrated significant reductions in HbA1c compared to placebo and dulaglutide across various dosage levels. A notable proportion of patients achieved HbA1c targets of less than 7.0% and at least 6.5% with LY3298176. Changes in fasting plasma glucose, body weight, waist circumference, and total cholesterol also showed favorable outcomes with LY3298176 compared to placebo and dulaglutide. Serious adverse events were infrequent, occurring in 4% of participants across all treatment groups. These findings were consistent at both the 12-week and 26-week mark for secondary outcomes. |
|
Rosenstock et al., 2021 (13) |
Between June 3, 2019, and Oct 28, 2020, a total of 705 individuals were screened for eligibility. 478 participants meeting eligibility criteria were randomized into four groups: tirzepatide 5 mg (n=121), tirzepatide 10 mg (n=121), tirzepatide 15 mg (n=121), and placebo (n=115). Baseline characteristics included mean HbA1c of 7.9% (63 mmol/mol), mean age of 54.1 years (SD 11.9), 48% women, diabetes duration of 4.7 years, and mean body-mass index of 31.9 kg/m². At 40 weeks, all tirzepatide doses were superior to placebo in reducing HbA1c, fasting serum glucose, bodyweight, and achieving HbA1c targets of less than 7.0% (<53 mmol/mol) and less than 5.7% (<39 mmol/mol). Tirzepatide induced dose-dependent weight loss ranging from 7.0 to 9.5 kg. The most frequent adverse events with tirzepatide were mild to moderate gastrointestinal events, including nausea, diarrhea, and vomiting. No clinically significant or severe hypoglycemia was reported with tirzepatide, and one death occurred in the placebo group. |
|
Heise et al., 2023 (14) |
Tirzepatide treatment showed substantial decreases in body weight in contrast to both placebo and semaglutide, leading to more significant reductions in fat mass. Both tirzepatide and semaglutide notably decreased appetite compared to placebo. There were no discernible differences in appetite scores and reductions in energy intake between tirzepatide and semaglutide treatments. |
|
Pirro et al., 2022 (15) |
At the 26-week mark, a higher dosage of tirzepatide influenced a group of metabolites and lipids linked with insulin resistance, obesity, and the risk of future type 2 diabetes. Various metabolites such as branched-chain amino acids, glutamate, 3-hydroxyisobutyrate, branched-chain ketoacids, and byproducts like 2-hydroxybutyrate exhibited decreases in comparison to baseline and placebo levels. The reductions observed with tirzepatide were notably more significant when compared to dulaglutide, and they were directly correlated with decreases in HbA1c, homeostatic model assessment 2-insulin resistance (HOMA2-IR) indices, and proinsulin levels. In line with these changes in metabolites, triglycerides and diglycerides were notably reduced compared to baseline, dulaglutide, and placebo, particularly favoring shorter and highly saturated species. |
BMI: Body Mass Index
However, it's essential to note the presence of significant heterogeneity among the studies, as indicated by an I^2 of 99.2% and a tau^2 of 0.6040. This suggests substantial variability in effect sizes among the included studies. Despite this heterogeneity, the findings remained robust, with consistent reductions in HbA1c levels observed across all studies (Figure 3).
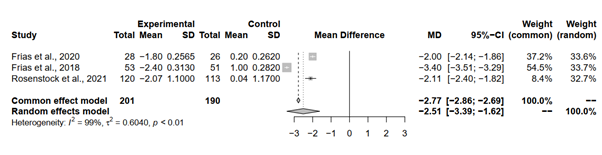
Figure 3: Forest plot of tirzepatide 15 mg compared to placebo on HbA1c level
Tirzepatide 15 mg compared to placebo on fasting blood glucose (FBG) level
Pooling the results under both the common effect and random effects models revealed consistent and statistically significant reductions in FBG levels with tirzepatide 15 mg across all analyses. The MD estimates, coupled with narrow 95% CIs, indicate a robust and reliable effect of Tirzepatide on FBG levels. Interestingly, both models yielded identical MD estimates of -62.78 (95%-CI [-67.9269; -57.6337]), underpinning the consistency and strength of the observed effect.
Heterogeneity analysis aimed to explore the variability in effect sizes among the included studies. Remarkably, minimal heterogeneity was observed, with an I^2 of 0.0% and a tau^2 of 0, suggesting that the observed differences in effect sizes are likely attributable to chance. The non-significant p-value (p = 0.5508) obtained from the Q-test further corroborates the absence of substantial heterogeneity, reinforcing the robustness and reliability of the meta-analytical findings (Figure 4).
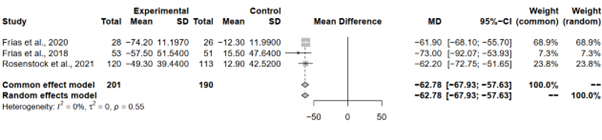
Figure 4: Forest plot of tirzepatide 15 mg compared to placebo on FBG level
Tirzepatide 15 mg compared to placebo on waist circumference
The meta-analysis aimed to assess the impact of Tirzepatide 15 mg compared to placebo on waist circumference across three studies. Each study contributed valuable insights into the efficacy of tirzepatide in modifying waist circumference.
Pooling the results under both the common effect and random effects models unveiled consistent and statistically significant reductions in waist circumference with Tirzepatide 15 mg across all analyses. While both models yielded significant MD estimates, the random effects model exhibited a slightly wider CI range. Specifically, the common effect model yielded an MD estimate of -8.24 (95%-CI [-8.7252; -7.7487]), and the random effects model provided an estimate of -8.60 (95%-CI [-15.5491; -1.6410]). This implies some variability in effect sizes among the studies, although the overall trend towards reduced waist circumference with tirzepatide remains robust.
Heterogeneity analysis aimed to explore the variability in effect sizes among the included studies. Notably, high heterogeneity was observed, with an I^2 of 99.6% and a tau^2 of 37.0876, indicating substantial variability in effect sizes. The significant p-value obtained from the Q-test further corroborates the presence of heterogeneity among the studies (p < 0.0001) (Figure 5).
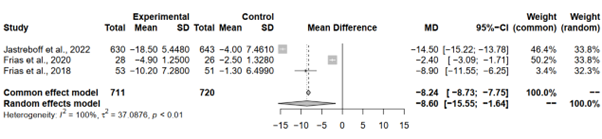
Figure 5: Forest plot of tirzepatide 15 mg compared to placebo on waist circumference
Tirzepatide 15 mg compared to placebo on changes in body weight
The meta-analysis sought to evaluate the impact of tirzepatide 15 mg compared to placebo on changes in body weight across five studies. Each study provided essential data regarding the magnitude of weight change associated with Tirzepatide treatment.
Both the common effect and random effects models demonstrated significant reductions in body weight change with Tirzepatide 15 mg compared to placebo. While the common effect model yielded a MD estimate of -10.3625 (95%-CI [-10.6780; -10.0471]), the random effects model provided an estimate of -10.7852 (95%-CI [-14.8264; -6.7439]). Despite slight differences in MD estimates between the models, both underscored the robustness of the observed effect. However, the presence of high heterogeneity, indicated by an I^2 of 99.4% and significant p-value from the Q-test (p < 0.0001), suggests potential variability in treatment response or study characteristics across the included studies (Figure 6).
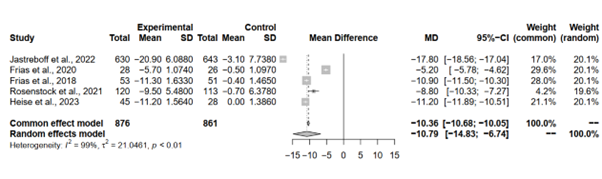
Figure 6: Forest plot of tirzepatide 15 mg compared to placebo on changes in body weight
Tirzepatide 15 mg compared to placebo on serious adverse events rate
The meta-analysis scrutinized the occurrence of serious adverse events among individuals treated with tirzepatide 15 mg compared to those administered placebo across three studies. Each study contributed crucial data regarding the relative risk of experiencing serious adverse events under tirzepatide treatment. Notably, Jastreboff et al., 2022, showcased a RR of 0.7423, suggesting a 25.77% reduction in the risk of such events compared to placebo, albeit not statistically significant, with a 95% CI spanning [0.4771; 1.1547]. Conversely, Frias et al., 2018, exhibited an RR of 0.9623, indicating a negligible difference in risk between Tirzepatide and placebo groups, with a wide 95% CI encompassing [0.1408; 6.5760]. Similarly, Rosenstock et al., 2021, reported an RR of 0.3139, suggesting a substantial reduction in risk with tirzepatide treatment, though the CI ranged widely from [0.0331; 2.9738]. Importantly, Frias et al., 2020, lacked sufficient data to compute RR, hence marked as not applicable.
Pooling the results under both the common effect and random effects models revealed a trend towards a lower risk of serious adverse events with tirzepatide 15 mg compared to placebo, albeit non-significant. Both models yielded identical RR estimates and 95% CIs, with p-values of 0.1432. Despite this trend, it's crucial to acknowledge the absence of significant heterogeneity among the studies, as indicated by an I^2 of 0.0% and a tau^2 of 0. This lack of heterogeneity enhances the reliability of the results, although caution is warranted due to the limited number of studies and events, as well as the wide confidence intervals observed in some instances (Figure 7).
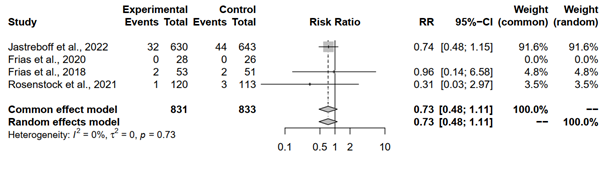
Figure 7: Forest plot of tirzepatide 15 mg compared to placebo on serious adverse events rate
Tirzepatide 15 mg compared to placebo on mortality rate
The meta-analysis assessed mortality rates between individuals receiving tirzepatide 15 mg and those administered a placebo across two studies. Notably, Jastreboff et al., 2022, reported a RR of 0.2552, indicating a potential reduction in mortality risk with tirzepatide, although the wide 95% CI [0.0286; 2.2766] encompassed 1, signifying uncertainty in the effect estimate. Similarly, Rosenstock et al., 2021, exhibited a RR of 0.3140, suggesting a similar trend towards reduced mortality risk with tirzepatide treatment, albeit with a wider CI [0.0129; 7.6283], indicating greater uncertainty in this effect estimate. On the other hand, Frias et al., 2020, lacked sufficient data to compute RR, thus being marked as not applicable.
Pooling the results under both the common effect model and random effects model yielded identical RR estimates and 95% CIs, with p-values of 0.1582. The RR estimate of 0.2727 implied a potential reduction in mortality risk with tirzepatide, although statistical significance was not reached at the conventional alpha level of 0.05. Despite this, the absence of significant heterogeneity, as indicated by an I^2 of 0.0% and a tau^2 of 0, enhances the reliability of the results (Figure 8).
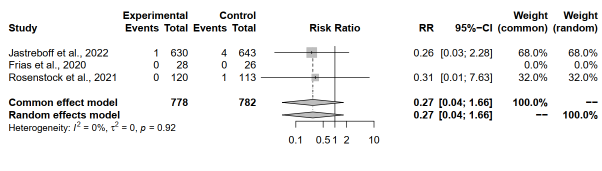
Figure 8: Forest plot of tirzepatide 15 mg compared to placebo on mortality rate
Publication Bias Assessment
Tirzepatide 15 mg compared to placebo on HbA1c level
The Egger's regression test, a pivotal tool in meta-analysis, was conducted to assess funnel plot asymmetry, aiming to illuminate potential publication bias or systematic heterogeneity within the meta-analysis. Employing a mixed-effects meta-regression model with the standard error as the predictor, the test yielded significant results. Specifically, a z-value of 1.9632 was obtained alongside a corresponding p-value of 0.0496, meeting conventional thresholds for statistical significance.
The statistically significant p-value (p = 0.0496) underscores the presence of funnel plot asymmetry, indicating potential biases in the included studies or systematic differences impacting effect size estimates. This outcome warrants a cautious interpretation of the meta-analysis findings, as the observed asymmetry could distort the true effect size estimate. Furthermore, the Egger's regression test provided a limit estimate, revealing an intercept of -12.0938 with a confidence interval ranging from -18.0782 to -6.1094 as the standard error approaches zero (Figure 9).
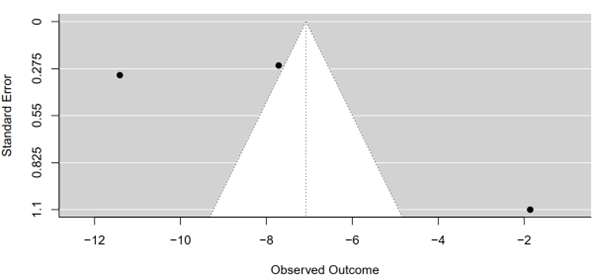
Figure 9: Funnel plot of tirzepatide 15 mg compared to placebo on HbA1c level
Tirzepatide 15 mg compared to placebo on FBG level
The meta-analysis comparing FBG levels between tirzepatide 15 mg and placebo groups across the included studies was subjected to a regression test for funnel plot asymmetry.
The test outcome revealed a z-value of 0.1134 and a corresponding p-value of 0.9097. This p-value, well above the conventional significance threshold of 0.05, suggests a lack of statistical evidence for funnel plot asymmetry. Consequently, there is no indication of substantial publication bias or systematic heterogeneity influencing the meta-analysis findings (Figure 10).
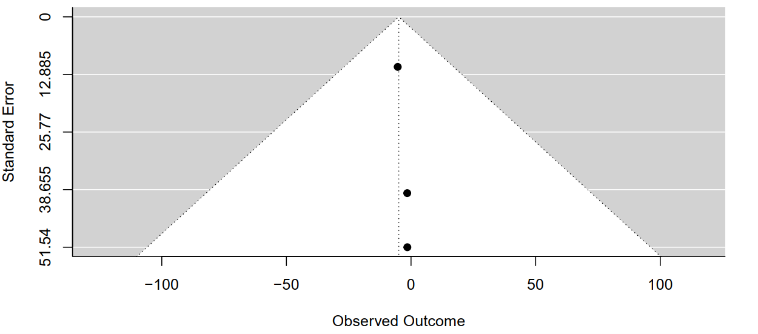
Figure 10: Funnel plot of tirzepatide 15 mg compared to placebo on FBG level
Tirzepatide 15 mg compared to placebo on waist circumference
The results of Egger's regression test for funnel plot asymmetry in the meta-analysis examining waist circumference between tirzepatide 15 mg and placebo groups reveal no significant evidence of asymmetry. Conducted within a mixed-effects meta-regression model, the test yielded a z-value of 0.0136 with a corresponding p-value of 0.9892, indicating no statistically significant asymmetry. This suggests a lack of substantial publication bias or systematic heterogeneity influencing the meta-analysis findings. Furthermore, the limit estimate, computed as -1.8831 with a confidence interval spanning from -5.6292 to 1.8630, underscores uncertainty regarding the estimated bias in the effect size as the standard error approaches zero. In essence, the results of Egger's regression test suggest that the meta-analysis conclusions regarding waist circumference are robust and not significantly affected by publication bias or systematic heterogeneity (Figure 11).
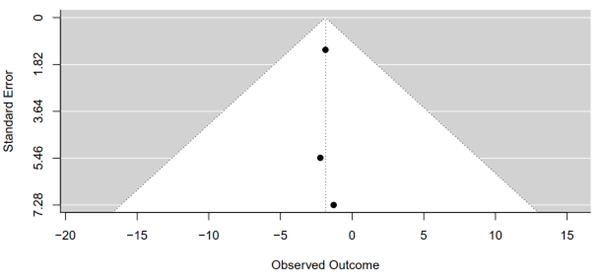
Figure 11: Funnel plot of tirzepatide 15 mg compared to placebo on waist circumference
Tirzepatide 15 mg compared to placebo on changing in body weight
The test revealed a z-value of 0.6827 and a corresponding p-value of 0.4948. These results indicate a lack of statistical evidence for funnel plot asymmetry at the conventional significance level of 0.05, suggesting minimal influence of publication bias or systematic heterogeneity on the meta-analysis outcomes concerning body weight changes (Figure 12).
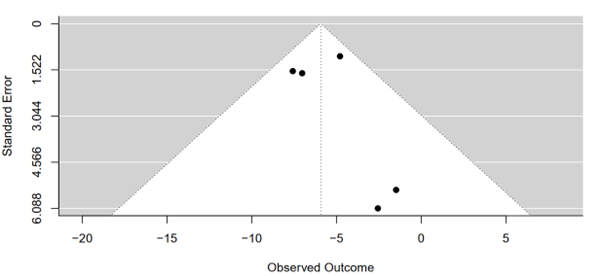
Figure 12: Funnel plot of tirzepatide 15 mg compared to placebo on changing in body weight
Tirzepatide 15 mg compared to placebo on serious adverse events rate
The Rank Correlation Test for Funnel Plot Asymmetry, employing Kendall's tau statistic, was conducted to scrutinize the presence of publication bias in the meta-analysis results. The computed Kendall's tau value of 0.6667 suggests a moderate to strong positive correlation between effect sizes and their corresponding standard errors in the funnel plot. However, the associated p-value of 0.3333 indicates that this correlation is not statistically significant at the conventional significance level of 0.05. Consequently, there is insufficient evidence to reject the null hypothesis of no correlation between effect sizes and their standard errors. These findings suggest that any observed asymmetry in the funnel plot is likely due to chance rather than systematic bias or publication bias. Thus, the meta-analysis results regarding serious adverse events between tirzepatide 15 mg and placebo groups are less likely to be influenced by publication bias, enhancing the confidence in the validity of the findings (Figure 13).
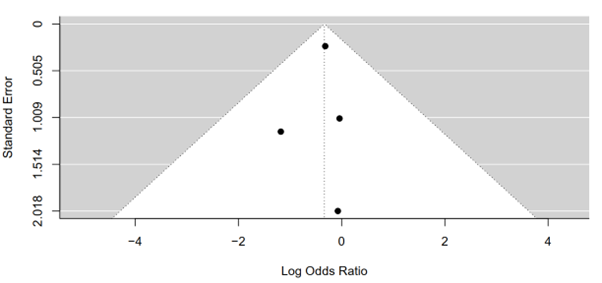
Figure 13: Funnel plot of tirzepatide 15 mg compared to placebo on serious adverse events rate
Tirzepatide 15 mg compared to placebo on mortality rate
The test yielded a Kendall's tau value of 1.0000, indicating a perfect positive correlation between effect sizes and their corresponding standard errors in the funnel plot. This suggests that larger studies tend to have larger effect sizes, while smaller studies tend to have smaller effect sizes, as expected. However, the associated p-value of 0.3333 indicates that this correlation is not statistically significant at the conventional significance level of 0.05. Therefore, there is insufficient evidence to reject the null hypothesis of no correlation between effect sizes and their standard errors. These findings imply that any observed asymmetry in the funnel plot is likely due to chance rather than systematic bias or publication bias. Thus, the results of the meta-analysis comparing mortality rates between tirzepatide 15 mg and placebo groups are less likely to be influenced by publication bias, bolstering confidence in the validity of the findings (Figure 14).
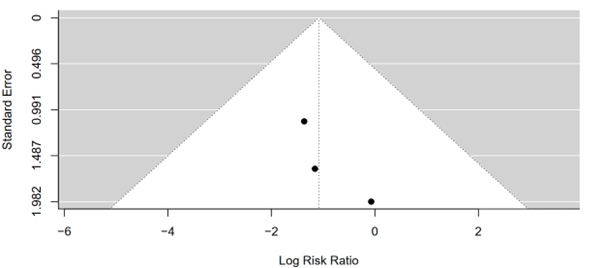
Figure 14: Funnel plot of tirzepatide 15 mg compared to placebo on mortality rate
Discussion
This comprehensive systematic review and meta-analysis thoroughly investigates the effectiveness of tirzepatide in managing both obesity and diabetes. Tirzepatide is defined as a dual GIP/GLP-1 receptor agonist. GLP-1 therapy is a well-known and established option for treating T2DM due to its effectiveness in controlling blood sugar levels, promoting weight loss, and providing favorable cardiovascular outcomes. It's often recommended as an early intervention in the treatment process. On the contrary, GIP was previously considered ineffective as a therapy for lowering blood glucose because studies showed no significant insulin response when given at high doses to individuals with T2DM. Recent studies indicate that when GLP-1 and GIP are administered concurrently, they exhibit a synergistic effect, eliciting a much stronger response in insulin and glucagon compared to when each hormone is administered independently. This discovery has spurred the development of a dual GIP/GLP-1 receptor agonist, commonly referred to as a 'twincretin'. Tirzepatide, a novel medication within this category, is composed of a synthetic peptide comprising 39 amino acids, modeled after the natural sequence of GIP. Both preclinical and early clinical trials phase 1 and 2 have demonstrated that tirzepatide effectively reduces blood sugar levels and induces weight loss, with side effects resembling those observed with established GLP-1 receptor agonists (16). This innovative medication for diabetes is a synthetic peptide derivative of human GIP hormone. It contains a C20 fatty-diacid segment, allowing it to bind to albumin using acylation technology. This enables a weekly subcutaneous injection, aligning with its approximate half-life of five days, to deliver an appropriate dose of the drug (17).
Tirzepatide has received approval for diabetes management in the USA, Europe, and the UAE. Clinical trials of this once-weekly injection have shown outstanding effectiveness in regulating blood sugar levels, frequently achieving normalization, and in reducing body weight to a degree that could substantially influence the course of T2DM, potentially even inducing remission. The substantial impact of tirzepatide on blood sugar levels and weight loss marks a new chapter in diabetes management, suggesting that a significant portion of patients could achieve established treatment goals (18). Comparative analyses conducted in individuals with T2DM indicate that tirzepatide typically offers superior management of blood sugar levels compared to selective GLP-1 receptor agonists such as dulaglutide and semaglutide, potentially due to an amplified insulin reaction to glucose. This heightened response could be linked to tirzepatide's activation of GIP receptors. However, earlier studies have demonstrated minimal activation of beta cells in T2DM patients receiving native GIP. However, it's plausible that tirzepatide’s prolonged exposure could amplify GIP receptor stimulation compared to acute treatments in older studies. Additionally, intensive insulin therapy over four weeks has been found to partially restore GIP's insulinotropic action, suggesting that similar effects may occur with tirzepatide treatment over several weeks. The concurrent activation of GLP-1 receptors may also play a role, given the known interactions between GIP and GLP-1 signaling pathways. If tirzepatide-induced GIP receptor stimulation indeed enhances beta-cell secretion, it suggests a time-dependent process that could restore GIP's effectiveness as an insulin secretagogue even in advanced type 2 diabetes. However, confirming these hypotheses requires detailed mechanistic studies (18).
Tirzepatide made key improvements which include alterations to peptide backbone residues to activate the GIP receptor, elongation of the C-terminus with a sequence resembling exenatide, and addition of a fatty acid side chain to prolong its half-life to 116.7 hours. Its hepatoprotective effects are noteworthy. The structural foundation of tirzepatide, along with its functional versatility, has been documented, shedding light on its physiological mechanisms in managing T2DM. Pharmacokinetic (PK) investigations in healthy volunteers across doses from 0.25 to 15 mg demonstrate dose-proportional peak plasma concentrations (Cmax) ranging from 26 to 874 ng/mL. Peak concentration (Tmax) typically occurs 1–2 days after administration, with an average half-life of five days, favoring weekly dosing. Steady-state exposure with weekly dosing is attained after 4 weeks, with an accumulation index of 1.6. In T2D patients receiving a 15 mg dose, Cmax is 1250 ng/mL, with Tmax observed at 24 hours. Further PK investigations in healthy volunteers over 28 days, involving dose escalation up to 10 mg followed by an oral glucose tolerance test, reveal significantly improved glucose responses compared to placebo, along with an average reduction in body weight of approximately 4.05 kg in subjects receiving the 10 mg dose (17).
By utilizing evidence from recent randomized trials, the results from both random and common effects models demonstrate statistically significant findings. This strongly indicates tirzepatide's efficacy in reducing HbA1c levels (p < 0.0001). The MD estimates, alongside narrow 95% CIs, reinforce the robust and consistent effect of tirzepatide on FBG levels. Notably, both models produce identical MD estimates (-62.78; 95% CI [-67.9269; -57.6337]), highlighting the reliability of the observed effect. Significant reductions in waist circumference are consistently observed with Tirzepatide 15 mg across all analyses, as well as significant reductions in body weight compared to placebo. While there are slight disparities in MD estimates between the common and random effects models, both affirm the strength of the effect observed. Although there is a trend towards a lower risk of serious adverse events with tirzepatide 15 mg compared to placebo, statistical significance is not achieved. Nonetheless, identical relative risk estimates and 95% CIs (p = 0.1432) suggest a potential reduction in adverse events with tirzepatide. The overall findings suggest tirzepatide to be a safe therapeutic agent with minimal reported adverse events. Additionally, the relative risk estimate for overall mortality (0.2727) implies a potential reduction in mortality risk with tirzepatide, although statistical significance is not attained at the conventional alpha level of 0.05. The absence of significant heterogeneity, indicated by an I² of 0.0% and a tau² of 0, further enhances the reliability of these results.
Similar to our findings Sinha et al. concluded in their review that the significant weight loss ranging from 5.4 to 12.9 kg and notable enhancements in glycemic control with reductions in HbA1c ranging from 1.87% to 3.02% observed in the SUPRASS program with tirzepatide indicate a promising shift in the landscape of T2DM pharmacotherapy. This suggests that achieving weight loss of ≥10% and even ≥15%, alongside reaching an HbA1c level of ≤6.5%, is now a feasible goal, irrespective of the background glucose-lowering medications and even in individuals with over 10 years of T2DM duration. Across all clinical trials comparing tirzepatide with active comparators such as basal insulins and once-weekly GLP-1 receptor agonists like dulaglutide up to 1.5 mg and semaglutide 1 mg, tirzepatide demonstrated superior efficacy not only in improving glycemic levels but also in achieving clinically significant weight loss and enhancing various cardiometabolic risk factors (9). Likewise, findings of a meta-analysis by Tan et al. highlighted tirzepatide at 5 mg exhibited superior weight loss compared to both placebos mean difference: -12.47 kg, 95% confidence interval: -13.94 kg to -11.00 kg and semaglutide (n = 1409, mean difference: -1.90 kg, 95% confidence interval: -2.97 kg to -0.83 kg), with a dose-dependent trend observed for the 10 mg and 15 mg doses. The potential of tirzepatide as a treatment for weight loss in overweight and obese individuals is notable, particularly due to its relatively low occurrence of adverse effects when compared to alternative weight loss medications. With its capability to tackle various components of metabolic syndrome concurrently, tirzepatide emerges as a promising choice within the landscape of weight loss treatments (19). Another meta-analysis by Tang et al. indicated that treatment with tirzepatide led to reductions in HbA1c (weighted mean difference: -1.07%; 95% [CIs]: -1.44, -0.56), fasting serum glucose (weighted mean difference: -21.50 mg/dl; 95% CI: -34.44, -8.56), body weight (weighted mean difference: -7.99 kg; 95% CI: -11.36, -4.62), and blood pressure, as well as improvements in fasting lipid profiles, without an increase in hypoglycemia, whether used as a monotherapy or an add-on therapy. The utilization of tirzepatide correlated with an elevated likelihood of gastrointestinal side effects, especially when utilized as an adjunctive treatment, although no increased risk was noted concerning pancreatitis or cholelithiasis. Furthermore, tirzepatide displayed a dose-dependent correlation with decreasing HbA1c and body weight, alongside an increased occurrence of nausea and vomiting (20). Similarly, in this study, the predominant adverse effects reported were mild to moderate gastrointestinal disturbances, primarily consisting of nausea, vomiting, and diarrhea.
A systematic review by Lin et al. further highlighted that tirzepatide has shown promise in reducing body weight among patients with T2DM and obesity. It presents as a potentially effective treatment for weight loss; however, caution is warranted due to possible gastrointestinal reactions. Among adverse events observed, gastrointestinal issues were the most frequent across all groups. The occurrence of gastrointestinal adverse events such as diarrhea, nausea, vomiting, and decreased appetite was comparable between tirzepatide and GLP-1 receptor agonists. Nonetheless, when compared to either a placebo or basal insulin, tirzepatide was associated with increased likelihoods of experiencing diarrhea, nausea, vomiting, decreased appetite, and constipation (21).
Adding further in this context Qin et al. reported in their meta-analysis findings that treatment with tirzepatide has demonstrated the potential for significant and enduring weight loss, with good tolerability and safety, offering a fresh and valuable approach to managing weight over the long term. Additionally, Tirzepatide has shown notable effectiveness in lowering blood pressure, blood glucose levels, and improving lipid profiles. Regarding safety, gastrointestinal side effects were commonly reported across all three doses of tirzepatide, typically presenting as mild to moderate and temporary (22). Kaore et al. similarly concluded in their meta-analysis results that tirzepatide improves the control of blood glucose levels and facilitates weight reduction in individuals with T2DM, showing beneficial impacts on lipid profiles, without increasing the risk of hypoglycemia or cardiovascular events. Nevertheless, it is linked to a decreased occurrence of hypoglycemia but a higher prevalence of gastrointestinal adverse effects like nausea, vomiting, and diarrhea (23). The findings from our study align closely with the conclusions drawn from recent research documented in literature. This harmony between our results and those of other studies lends weight to the consistency and reliability of the evidence surrounding tirzepatide. Notably, our investigation underscores the robustness of tirzepatide's safety and effectiveness in managing both diabetes and obesity. It's worth highlighting that despite its effectiveness, tirzepatide is linked to controllable gastrointestinal side effects, mainly described as mild to moderate, which may include occurrences of nausea, vomiting, and diarrhea. Evaluating this aspect is essential in understanding the overall risk-benefit balance of tirzepatide as a treatment choice for individuals with diabetes and obesity. The collective body of evidence, including our study, strongly supports the favorable role of tirzepatide in addressing these prevalent and challenging health conditions. Through various studies and research endeavors, it has become increasingly evident that tirzepatide offers a valuable therapeutic option in addressing these complex conditions. Its demonstrated safety profile and efficacy contribute significantly to its potential as a cornerstone in the treatment regimen for individuals grappling with diabetes and obesity.
This systematic review and meta-analysis, which concentrated on evaluating the efficacy and safety of tirzepatide in treating type 2 diabetes and/or obesity through clinical trials, presents several notable strengths. Firstly, it allowed for a comprehensive evaluation of all relevant studies, specifically clinical trials of recent times, providing a thorough overview of the available evidence. By combining data from multiple trials, meta-analysis increases statistical power, enhancing the precision of estimates regarding tirzepatide's effects. Moreover, adherence to strict inclusion criteria and systematic search methods reduces bias in study selection and data extraction, ensuring a more objective assessment. Quantitative synthesis enabled the estimation of overall treatment effects and the exploration of potential sources of heterogeneity. Pooling data from diverse studies enhanced the generalizability of findings, providing robust evidence applicable to various populations and settings. By synthesizing the best available evidence, this review supports evidence-based decision-making processes for healthcare providers, policymakers, and patients considering tirzepatide as a treatment option. Finally, meta-analysis allowed for the assessment of publication bias, helping to identify and mitigate potential distortions in the literature. Overall, leveraging these strengths contributes to a comprehensive understanding of tirzepatide's efficacy and safety, facilitating evidence-based clinical practice and healthcare decision-making.
Limitations and future research directions
However, it's important to acknowledge certain limitations within this study that should be taken into account when interpreting the findings. Firstly, the analysis only includes data on tirzepatide dosage of 15 mg. This limitation suggests that the generalizability of our results may be restricted to this specific dosage. Therefore, it's essential to recognize that the findings might not fully represent the effects of tirzepatide at other dosages. Moreover, potential heterogeneity among intrinsic characteristics of the included studies, publication bias that may skew results toward positive outcomes, and a paucity of long-term data to evaluate the durability of tirzepatide's effects further define the limitations of this study. Future research recommendations include addressing several key areas. Conducting direct comparative trials between tirzepatide and other treatments is crucial for clearer insights into its relative efficacy and safety. Ensuring adequate representation of diverse patient subgroups in studies will improve the generalizability of the findings. Additionally, accounting for confounding factors within included trials is essential to reduce potential biases. When extrapolating trial results to real-world clinical practice settings, it's important to consider the evolving landscape of diabetes and obesity management. Extending follow-up periods in trials will help assess long-term outcomes related to glycemic control, weight management, and cardiovascular health. Detailed subgroup analyses based on demographic variables, baseline characteristics, and comorbidities are necessary to understand different population responses to treatment. Comparing tirzepatide with existing therapies through direct comparisons or network meta-analyses will inform treatment selection. Exploring patient-reported outcomes can provide valuable insights into treatment satisfaction and adherence. Investigating the underlying mechanisms of tirzepatide's action through translational research and biomarker analyses is needed for a deeper understanding of its effects. Finally, continuously monitoring safety profiles and integrating real-world evidence studies and economic evaluations will provide a comprehensive understanding of tirzepatide's clinical benefits and support evidence-based decision-making processes.
Conclusion
This systematic review and meta-analysis have provided valuable insights into the efficacy and safety of tirzepatide in the management of T2DM and obesity. The findings underscore tirzepatide's significant potential as a treatment option, particularly in improving glycemic control and inducing weight loss with minimal adverse events reported making it a safe and effective option in clinical practice. However, it's important to acknowledge the limitations of the available evidence, including the need for further research to explore long-term outcomes, conduct subgroup analyses, and compare tirzepatide with other treatments. Additionally, ongoing monitoring of safety profiles and integration of real-world evidence studies are essential for a comprehensive understanding of tirzepatide's clinical utility.
Disclosures
Author Contributions
The author has reviewed the final version to be published and agreed to be accountable for all aspects of the work.
Ethics Statement
Not applicable
Consent for publications
Not applicable
Data Availability
All data is provided within the manuscript.
Conflict of interest
The author declare no competing interest.
Funding
The author has declared that no financial support was received from any organization for the submitted work.
Acknowledgements
Not Applicable