Volume 4, Issue 8
August 2024
Assessing the Role of Pulmonary Vasodilators in Emergency Medicine: A Systematic Review
Malak Fahad Alotaibi, Nouf Sajer Alghorry
DOI: http://dx.doi.org/10.52533/JOHS.2024.40805
Keywords: pulmonary, vasodilators, management, emergency, medicine
Background: Pulmonary vasodilators play a crucial role in emergency medicine by providing rapid relief and stabilization in acute pulmonary conditions characterized by increased pulmonary vascular resistance and hemodynamic instability. These medications act to dilate blood vessels in the lungs, thereby reducing pulmonary artery pressure and improving blood flow to optimize oxygenation and cardiac function.
Methodology: In this systematic review we aim to investigate the role of pulmonary vasodilators in emergency medicine settings, focusing on their efficacy and safety in managing various conditions. The inclusion criteria for this systematic review encompassed studies published in peer-reviewed journals focusing on human subjects treated in emergency departments using pulmonary vasodilators. The Newcastle-Ottawa Scale was employed to assess the quality and potential biases in the observational studies included in this review, while for randomized trials, RoB 2: A revised Cochrane risk-of-bias tool for randomized trials was used.
Results: We incorporated a total of 8 studies encompassing 1648 patients, published between 2002 and 2022. Diverse conditions reported in emergency settings were hypoxemic respiratory failure, sickle cell disease, eczema, chronic sinusitis and gastroesophageal reflux, asthma, respiratory symptoms of SARS-CoV-2 infection, acute pulmonary embolism, pulmonary edema, pulmonary arterial hypertension, sepsis, and acute respiratory distress syndrome. Inhaled epoprostenol, nitric oxide, bronchodilator, and intravenous nitroglycerine, along with bosentan and sildenafil, were used for the management of these conditions. The outcomes varied, as the majority of the included studies reported successful management and improvement in the condition, while few other studies did not observe any significant differences.
Conclusion: The review highlights the significant impact of pulmonary vasodilators in the management of various conditions encountered or treated in emergency medicine settings. Future studies should focus on personalized approaches to therapy, alternative delivery methods, and the impact of pulmonary vasodilators on patient-centred outcomes, ultimately informing evidence-based practice and enhancing patient care in emergency medicine settings.
Introduction
Pulmonary vasodilators are medications designed to relax and widen the blood vessels within the lungs, specifically targeting the pulmonary circulation. By dilating these blood vessels, pulmonary vasodilators reduce pulmonary vascular resistance, which is the resistance to blood flow within the pulmonary arteries. This leads to improved blood flow and oxygenation in the lungs (1). There are several classes of pulmonary vasodilators, including inhaled nitric oxide, prostacyclin analogs, phosphodiesterase inhibitors, and endothelin receptor antagonists. Each class acts through different mechanisms to achieve vasodilation. For instance, inhaled nitric oxide directly targets smooth muscle cells in the pulmonary vasculature to induce relaxation, while prostacyclin analogs stimulate prostacyclin receptors on endothelial cells, leading to vasodilation and inhibition of platelet aggregation (2). These medications are used in the management of various pulmonary conditions characterized by increased pulmonary vascular resistance, such as acute pulmonary embolism, acute exacerbations of pulmonary hypertension, and acute respiratory distress syndrome. Pulmonary vasodilators can help improve oxygenation, reduce right ventricular strain, and stabilize hemodynamics in these acute settings. However, their use requires careful monitoring due to potential side effects such as systemic hypotension and rebound pulmonary hypertension (3).
Pulmonary vasodilators play a crucial role in emergency medicine by providing rapid relief and stabilization in acute pulmonary conditions characterized by increased pulmonary vascular resistance and hemodynamic instability. These medications act to dilate blood vessels in the lungs, thereby reducing pulmonary artery pressure and improving blood flow to optimize oxygenation and cardiac function. While emergency medicine encompasses a broad spectrum of conditions, the role of pulmonary vasodilators is particularly prominent in managing acute pulmonary embolism, acute exacerbation of pulmonary hypertension, and severe acute respiratory distress syndrome (4).
In the context of acute pulmonary embolism, which is a potentially life-threatening condition resulting from obstruction of the pulmonary arteries by emboli, pulmonary vasodilators can offer several benefits. In cases of massive or submassive pulmonary embolism associated with hemodynamic compromises, such as right ventricular strain or shock, pulmonary vasodilators like inhaled nitric oxide or prostacyclin analogs can rapidly reduce pulmonary vascular resistance (5). By dilating the pulmonary vasculature, these medications improve right ventricular function and decrease right ventricular afterload, thereby enhancing cardiac output and systemic perfusion. Additionally, pulmonary vasodilators may help redistribute blood flow to areas of the lung that are not affected by the embolism, optimizing gas exchange and reducing hypoxemia (6). Acute exacerbations of pulmonary arterial hypertension represent another critical scenario where pulmonary vasodilators are employed in emergency medicine. Pulmonary arterial hypertension is characterized by increased pulmonary vascular resistance, leading to right heart failure and systemic hypoxemia. During acute exacerbations, patients may experience worsening dyspnea, chest pain, and hemodynamic instability. Pulmonary vasodilators such as prostacyclin analogs (e.g., epoprostenol), endothelin receptor antagonists (e.g., bosentan), and phosphodiesterase inhibitors (e.g., sildenafil) are utilized to rapidly reduce pulmonary vascular resistance and alleviate right ventricular strain. These medications may be administered via various routes, including intravenous infusion, inhaled delivery, or oral administration, depending on the patient's clinical status and the urgency of treatment (7).
In the management of severe acute respiratory distress syndrome, characterized by diffuse alveolar damage and profound hypoxemia, pulmonary vasodilators have been investigated as adjunctive therapies to optimize oxygenation and lung mechanics. While the role of pulmonary vasodilators in acute respiratory distress syndrome remains controversial, studies have suggested potential benefits in improving ventilation-perfusion matching and reducing pulmonary shunting (8). Inhaled nitric oxide is the most extensively studied pulmonary vasodilator in acute respiratory distress syndrome, although its impact on clinical outcomes such as mortality remains uncertain. Nevertheless, in select cases of severe acute respiratory distress syndrome refractory to conventional therapies, the judicious use of pulmonary vasodilators may be considered to mitigate hypoxemia and improve respiratory mechanics (9). Despite their potential benefits, the use of pulmonary vasodilators in emergency medicine requires careful consideration of patient-specific factors, including hemodynamic stability, comorbidities, and potential contraindications. These medications can have significant side effects, including systemic hypotension, rebound pulmonary hypertension, and bleeding complications, particularly with prostacyclin analogs. Therefore, close hemodynamic monitoring and titration of doses are essential to optimize safety and efficacy (8).
The rationale for conducting a systematic review of the role of pulmonary vasodilators in emergency medicine stems from the critical importance of optimizing treatment strategies for acute pulmonary conditions. However, the evidence supporting their efficacy, safety, and optimal utilization in emergency settings remains heterogeneous and sometimes conflicting. By systematically reviewing the existing literature, this study aims to consolidate the available evidence, identify gaps in knowledge, and provide clinicians with comprehensive insights into the role of pulmonary vasodilators in emergency medicine. Additionally, given the potential variations in patient populations, treatment protocols, and outcomes across different emergency departments and healthcare settings, a systematic review offers a rigorous approach to synthesizing diverse evidence sources, enabling a more robust assessment of the clinical impact and applicability of pulmonary vasodilators in emergency care. Ultimately, the findings of this systematic review will help inform clinical practice guidelines, optimize patient management strategies, and guide future research efforts in this critical area of emergency medicine.
Material and Methods
Definition of Outcomes and Inclusion Criteria
In this systematic review, we aim to investigate the role of pulmonary vasodilators in emergency medicine settings, focusing on their efficacy and safety in managing various conditions. Our primary outcomes of interest include the impact of pulmonary vasodilators on hemodynamics, oxygenation, and clinical outcomes such as mortality, length of hospital stay, and adverse events.
The inclusion criteria for this systematic review encompassed studies published in peer-reviewed journals focusing on human subjects treated in emergency department using pulmonary vasodilators. Specifically, the review included investigations into reported outcomes and efficacy. Studies reporting treatment outcomes were considered. To maintain precision and reliability, the exclusion criteria involve the exclusion of animal studies, in vitro investigations, laboratory studies, and research with redundant findings. Additionally, abstract-only presentations, reviews, books, posters, theses, editorials, notes, letters, case reports, case series, and conference papers are excluded. Studies were selected based on inclusion and exclusion criteria by two independent authors. Any disagreement was settled by consensus among all authors.
Search Strategy
In February 2024, an extensive and systematic search of the literature was conducted across multiple electronic databases, including Scopus, Cochrane Library, Pubmed, and Web of Science. Additional eligible studies were identified by reviewing the references to all retrieved literature and reviewing articles addressing the outcomes of pulmonary vasodilators in emergency settings. Search terms were adapted as needed to suit the requirements of each database. The utilized search terms were subsequently adjusted based on the characteristics of each database.
Search terms were developed using a combination of medical subject headings (MeSH) and keywords related to pulmonary vasodilators, emergency medicine, and specific pulmonary conditions such as pulmonary embolism, pulmonary hypertension, and ARDS. The search strategy was tailored to each database's syntax and functionality, and Boolean operators (AND, OR) were used to combine search terms appropriately. Additionally, reference lists of included studies and relevant review articles were manually screened to identify additional studies that met the inclusion criteria.
Screening and Extraction
Articles with irrelevant titles were excluded from consideration. In the subsequent phase, both the full text and abstracts of the papers were meticulously reviewed to determine their compliance with the inclusion criteria. To streamline the process, titles and abstracts were organized, assessed, and scrutinized for any duplicate entries using reference management software (Endnote X8). To ensure the highest quality of selection, a dual screening approach was adopted, involving one screening for the evaluation of titles and abstracts, and another for the comprehensive examination of the entire texts. Once all relevant articles were identified, a structured extraction sheet was created to capture pertinent information aligned with our specific objectives.
Two separate researchers conducted the data extraction process independently. The gathered information included various study attributes like the author's name, publication year, country of origin, study design, sample size, and study duration. Additionally, details regarding participants' age and gender were also collected. Outcome measures included treatment efficacy, safety/adverse events, and hospital length of stay.
Quality Assessment
The Newcastle-Ottawa Scale (NOS) was employed to assess the quality and potential biases in the observational studies included in this review. The scale utilized in this assessment comprised 8 core elements categorized into 3 broad domains related to study quality: selection of study groups, comparability of groups, and ascertainment of outcomes. The first domain aimed to ascertain the representativeness of the exposed cohort. The second domain focused on determining if the study controlled for other variables. The third domain sought to evaluate the presence of bias in the measurement of the outcome. Each domain is assessed based on a set of criteria, and studies are awarded scores according to their fulfilment of these criteria. The maximum number of scores a study can receive is nine (10).
The risk of bias in randomized trials was assessed using the RoB 2: A revised Cochrane risk-of-bias tool for randomized trials (11). This tool evaluates multiple domains, including the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain is assessed for risk of bias, resulting in an overall judgment for each trial. Two independent reviewers evaluated the risk of bias for each included randomized trial according to the RoB 2 tool. Any discrepancies in assessments were resolved through discussion or consultation with a third reviewer. This systematic approach ensured a comprehensive evaluation of the methodological quality of randomized trials included in the review.
Results
Search Results
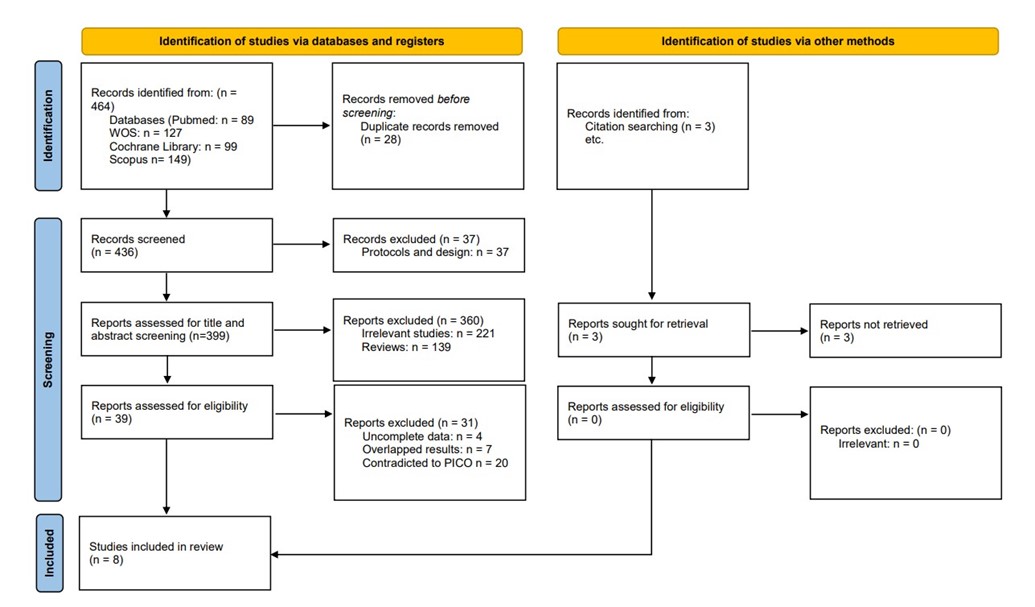
We executed the search methodologies outlined previously, resulting in the identification of a total of 464 citations, subsequently reduced to 436 following the removal of duplicates. Upon screening titles and abstracts, only 39 citations met the eligibility criteria for further consideration. Through full-text screening, this number was further refined to 8 articles aligning with our inclusion and exclusion criteria. Figure 1 provides an in-depth depiction of the search strategy and screening process.
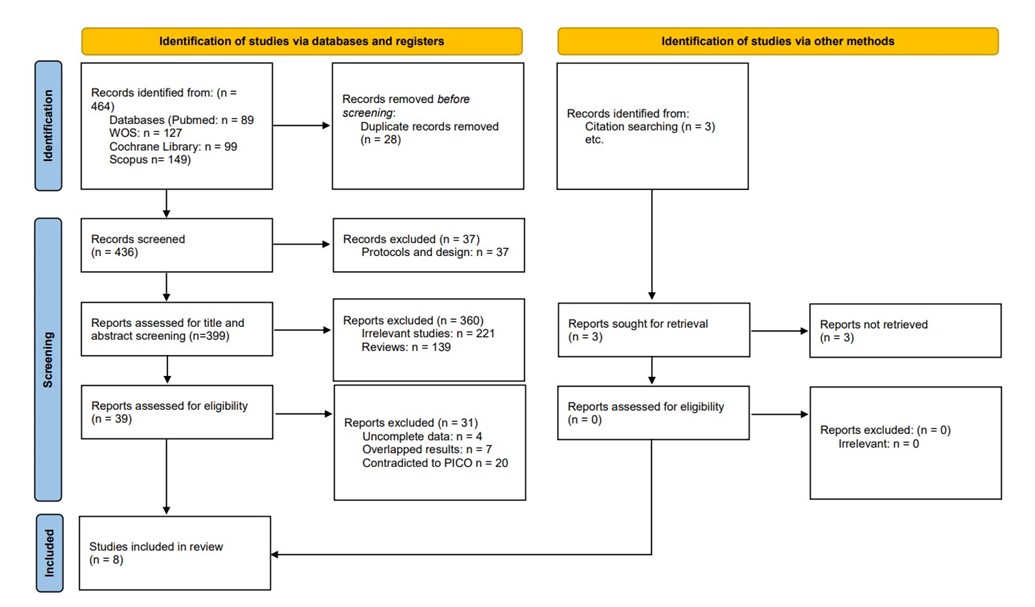
Figure 1: PRISMA flow diagram
Results of the Quality Assessment
The Cochrane risk of bias tool was employed to evaluate the methodological quality of four studies: Gladwin et al. (12), Kline et al. (13), Grunwell et al. (14), and Strickland et al. (15). Gladwin et al. (12) demonstrated the lowest risk across most domains, except for "Other bias," where it was rated high. Kline et al. (13) exhibited high risk in multiple areas, while Grunwell et al. (14) and Strickland et al. (15) presented a mix of unclear and high risks across different criteria. Overall, Gladwin et al. (12) appear to have the most robust methodology, while caution is warranted when interpreting the findings of the other studies due to potential biases identified by the Cochrane risk of bias tool (Table 1).
|
Table 1: Risk of bias assessment using the Cochrane Risk of Bias tool for RCTs |
|||||||
|
Study |
Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective reporting |
Other bias |
|
Gladwin MT, et al. (12) |
Low |
Unclear |
Low |
Low |
Low |
Low |
High |
|
Kline JA, et al. (13) |
High |
Unclear |
High |
High |
Low |
Low |
Low |
|
Grunwell JR, et al. (14) |
High |
High |
Unclear |
Unclear |
Low |
Low |
Low |
|
Strickland B, et al. (15) |
Unclear |
High |
Unclear |
Unclear |
Low |
Low |
Low |
The NOS was utilized to assess the methodological quality of four studies: Kinsella et al. (16), Angalakuditi et al. (17), Toomey et al. (18), and Houseman et al. (19). The scale evaluates studies based on three main criteria: selection of study groups, comparability of groups, and ascertainment of either the exposure or outcome of interest. Each study is awarded scores based on these criteria, with a higher number of scores indicating higher quality. Kinsella et al. (16) and Angalakuditi et al. (17) both received 4 scores, suggesting good quality in selection and ascertainment of outcome but no scores for comparability. Toomey et al. (18) received 5 scores, indicating decent quality, while Houseman et al. (19) received 6 scores, showing good selection and ascertainment of outcome but with some room for improvement in comparability (Table 2).
|
Table 2: Summary of the results of bias assessment of the observational studies using the modified Newcastle-Ottawa scale (NOS). |
||||
|
Study |
Selection |
Comparability |
Exposure/Outcome |
Overall star rating |
|
Kinsella JP, et al. (16) |
3 |
0 |
1 |
4 |
|
Angalakuditi M, et al. (17) |
3 |
0 |
1 |
4 |
|
Toomey D, et al. (18) |
4 |
0 |
1 |
5 |
|
Houseman BS, et al. (19) |
4 |
0 |
2 |
6 |
Characteristics of the included studies
We incorporated a total of 8 studies (12-19) encompassing 1648 patients, published between 2002 and 2022. Studies encompassed retrospective, prospective cohort, and randomized controlled trial designs, with participants ranging from newborns to adults. Some studies provided comprehensive demographic information, including mean age and gender distribution. A comprehensive summary of the baseline characteristics of these studies is illustrated in Table 3. Discrepancies in sample sizes across the included papers likely stem from differences in study objectives and inclusion criteria.
|
Table 3: Baseline Characteristics of included studies |
||||||||
|
Study |
Country |
Journal Publisher |
Year |
Study design |
Study period |
Total participants |
Mean age(years) |
Gender(M/F) |
|
Kinsella JP, et al. (16) |
USA |
AAP |
2002 |
Retrospective |
NR |
25 newborns |
NR |
NR |
|
Angalakuditi M, et al. (17) |
US |
Medical Economics |
2010 |
Retrospective |
2006-2008 |
706 |
56.6 ±17.6 |
52%/48% |
|
Gladwin MT, et al. (12) |
US |
JAMA |
2011 |
RCT |
2004-2008 |
150, iNo/placebo: 75/75 |
Median 24.2 |
100%/0% |
|
Kline JA, et al. (13) |
USA |
Emerg Med J. |
2013 |
Clinical trial |
|
8 |
56±16 |
50%/50% |
|
Grunwell JR, et al. (14) |
US |
J Allergy Clin Immunol Pract. |
2020 |
RCT |
NR |
630 |
12.8 ± 4.5 |
55.7%/44.3% |
|
Toomey D, et al. (18) |
USA |
Am J Emerg Med. |
2022 |
Retrospective chart review |
2018-2021 |
15 |
54 |
40%/60% |
|
Strickland B, et al. (15) |
USA |
Am J Emerg Med. |
2022 |
RCT |
2020 |
47 |
Treatment vs. placebo: 42/40 |
51.06%/48.94% |
|
Houseman BS, et al. (19) |
USA |
Am J Emerg Med |
2023 |
Retrospective |
2018 |
67 |
59 ±11 |
63% /37% |
NR: not reported, RCT: Randomized controlled trial
Study outcome measures
The utilization and administration of pulmonary vasodilators were assessed across various multidisciplinary conditions encountered in emergency medicine settings. Diverse conditions reported were hypoxemic respiratory failure, sickle cell disease, eczema, chronic sinusitis and gastroesophageal reflux, asthma, respiratory symptoms of SARS-CoV-2 infection, acute pulmonary embolism, pulmonary edema, pulmonary arterial hypertension, sepsis and acute respiratory distress syndrome. Inhaled epoprostenol, nitric oxide, bronchodilator, and intravenous nitroglycerine, along with bosentan and sildenafil, were used for the management of these conditions. The outcomes varied significantly, as some studies reported improvement in the condition while others did not observe any significant differences (Table 4). Kinsella et al. (16) favoured the use of nitric oxide as findings demonstrated that oxygenation improved after initiation of nitric oxide therapy at the referring institution and the overall survival rate was 84%, while Gladwin et al. disagreed as they did not observe any significant difference in time to crisis resolution between nitric oxide and placebo groups moreover; hospitalization length, pain scores, opioid usage, and acute chest syndrome rate showed no significant differences between nitric oxide and placebo groups (12, 16). Grunwell et al. demonstrated in their study findings that almost 6.7% of children and 9.3% of adolescents exhibited poor bronchodilator dose responsiveness, while emergency visits occurred in 29% of children and 21% of adolescents. Children and adolescents who achieved maximal bronchodilation with 720 mcg albuterol also had approximately 2-fold increased odds of an emergency visit and approximately 3-fold increased odds of hospitalization by 12 months (14). Strickland et al. described that among patients with confirmed COVID-19, 38% of those in the nitric oxide treatment group returned to the emergency department, compared to 27% in the control group. Hospitalization rates were 5% in the nitric oxide treatment group and 7% in the control group. One patient in the nitric oxide group required intubation (15). Additionally, Angalakuditi reported that the number of pulmonary arterial hypertension related per subject per month inpatient stays and emergency department visits, as well as per subject per month length of inpatient stays, were statistically similar between the subgroups (17). Furthermore, Kline et al. stated that inhaled NO reduced dyspnoea without adverse events in all patients with severe submassive pulmonary embolism. Each patient experienced a reduction in the numerical Borg score by more than 50% (13). Toomey et al. observed that there were no occurrences of clinically significant hypotension (MAP <65) in any of the cases where inhaled epoprostenol was administered and a majority of patients experienced a reduction in the requirement for fraction of inspired oxygen following the administration of inhaled epoprostenol (18). While Houseman et al. indicated that the rates of ICU admission, intubation, acute kidney injury at 48 hours, and hypotension were 37%, 21%, 13%, and 4% respectively, and in addition to receiving intravenous nitroglycerine, 73% of patients received non-invasive positive pressure ventilation, 48% received sublingual nitroglycerine or bolus nitroglycerin before high-dose nitroglycerin infusion, 58% received loop diuretics, and 34% received angiotensin-converting enzyme inhibitor (19).
Discussion
This research aimed to offer valuable perspectives on the administration of pulmonary vasodilators in emergency medicine. The results gleaned from the incorporated studies are heterogeneous, as some studies favoured the efficacious use of pulmonary vasodilators for various conditions ranging from chronic sinusitis and gastroesophageal reflux, asthma, and respiratory symptoms of SARS-CoV-2 infection to pulmonary embolism and edema, pulmonary arterial hypertension, sepsis, and acute respiratory distress syndrome, while a few other studies did not find any significant changes or differences. The majority of the included studies reported the use of nitric oxide, while administration of inhaled epoprostenol, bronchodilators, intravenous nitroglycerine, bosentan, and sildenafil was reported by each study.
|
Table 4: Summary findings of the included studies |
|||
|
Study |
Type of vasodilator |
Condition/Disease |
Findings |
|
Kinsella JP, et al. (16) |
Nitric Oxide |
Hypoxemic Respiratory Failure |
|
|
Gladwin et al. (12) |
Nitric Oxide |
Sickle cell disease |
|
|
Grunwell JR, et al. (14) |
Bronchodilator |
Eczema, chronic sinusitis and gastroesophageal reflux, asthma controller medication use, indoor exposures, and asthma-related healthcare utilization. |
|
|
Strickland B, et al. (15) |
Nitric Oxide |
respiratory symptoms of SARS-CoV-2 infection |
|
|
Angalakuditi. (17) |
Bosentan and sildenafil |
PAH |
|
|
Kline et al. (13) |
Nitric Oxide |
Acute pulmonary embolism |
|
|
Toomey et al. (18) |
Inhaled epoprostenol |
PE(47%), ARDS(20%), sepsis(20%) and others |
|
|
Houseman et al. (19) |
Intravenous nitroglycerin |
Acute pulmonary edema |
|
Findings from our study demonstrated that almost three studies reported efficacious use of nitric oxide for the management of patients in emergency medicine settings, as improvements in the condition were observed. It significantly improved oxygenation and was well tolerated with no or minimal adverse effects. Similarly, another study by Liu et al. reported that the use of inhaled nitric oxide in emergency medicine has been associated with notable improvements in oxygenation, making it a valuable intervention in managing acute pulmonary conditions (2). Inhaled nitric oxide is a selective pulmonary vasodilator that acts directly on vascular smooth muscle cells in the pulmonary circulation, leading to vasodilation and a reduction in pulmonary vascular resistance. Its rapid onset of action and localized effect make it particularly well-suited for acute pulmonary conditions where there is a need for immediate improvement in oxygenation and hemodynamics. One of the primary benefits observed after the administration of nitric oxide is the enhancement of oxygenation levels in patients experiencing respiratory distress. Inhaled nitric oxide acts as a potent pulmonary vasodilator, selectively dilating the pulmonary vasculature without affecting systemic circulation. This targeted vasodilation leads to a reduction in pulmonary vascular resistance, improved blood flow to ventilated lung regions, and subsequently enhanced oxygenation (20). Additionally, research indicates that by increasing the oxygen content in the blood, nitric oxide helps alleviate hypoxemia, which is often a critical concern in emergency medicine, particularly in conditions such as acute respiratory distress syndrome and acute pulmonary embolism (21).
Moreover, studies in the literature have demonstrated the importance of gradually decreasing and tapering off inhaled nitric oxide flow as oxygenation improves and the underlying pulmonary condition stabilizes. This approach helps prevent abrupt changes in pulmonary vascular tone, which could lead to rebound pulmonary hypertension or systemic hypotension. By closely monitoring the patient's oxygen saturation and hemodynamic parameters, healthcare providers can safely titrate nitric oxide therapy to maintain optimal oxygenation levels while avoiding adverse events (22). Furthermore, the judicious use of nitric oxide involves careful titration and monitoring to optimize its therapeutic benefits while mitigating potential adverse events. It is essential to adjust the flow of inhaled nitric oxide according to the patient's response and oxygenation status, aiming to achieve the desired effect while minimizing the risk of adverse effects (23).
In addition to improving oxygenation and preventing adverse events, the use of nitric oxide in emergency medicine has also been associated with a reduced incidence of mechanical ventilation in certain patient populations. In conditions such as acute respiratory distress syndrome, where severe hypoxemia and respiratory failure necessitate mechanical ventilation, nitric oxide therapy may help mitigate the need for invasive ventilation or reduce the duration of mechanical ventilation support (24). On the contrary, one of the studies included in this review highlighted that intubation was necessitated for one patient receiving nitric oxide therapy in the emergency department. However, evidence suggests that by enhancing oxygenation and pulmonary blood flow, nitric oxide can potentially improve lung compliance and reduce the severity of hypoxemia, thereby lessening the reliance on mechanical ventilation. This can be particularly beneficial in resource-limited settings or during surge situations where ventilator availability may be limited, or when avoiding invasive procedures is desirable to minimize patient risk and optimize resource allocation (25).
The efficacy of pulmonary vasodilators in pain reduction and addressing poor bronchodilator responses represents a fascinating area of exploration within respiratory medicine, offering potential therapeutic avenues for patients with diverse respiratory conditions. Pulmonary vasodilators, primarily used to dilate blood vessels in the lungs and reduce pulmonary vascular resistance, have been investigated for their ancillary effects beyond hemodynamic improvement. One aspect of interest is their potential role in pain reduction. Studies suggest that while the primary indication for pulmonary vasodilators lies in managing pulmonary hypertension and related cardiovascular conditions, emerging evidence suggests their utility in mitigating pain associated with certain pulmonary pathologies (26). Conditions such as pulmonary arterial hypertension and acute pulmonary embolism can manifest with chest pain due to increased pulmonary vascular resistance, right ventricular strain, or ischemia. By reducing pulmonary vascular resistance and improving right ventricular function, pulmonary vasodilators may alleviate the chest pain and discomfort associated with these conditions, enhancing patient comfort and quality of life (2).
Similarly, the results of our study indicate that for the emergent management of pulmonary embolism, nitric oxide and inhaled epoprostenol were utilized and resulted in the successful management of the condition, as the numerical Borg score in each patient was decreased by more than 50% and oxygen saturation improved from 93±5 to 97±3 while shock index decreased from 1.0±0.11 to 0.86±0.09 in the nitric oxide group while patients who received inhaled epoprostenol reduced demand for fraction of inspired oxygen was observed. These findings signify the beneficial role of pulmonary vasodilators in emergency settings; however, very few of the included studies also revealed that there were no significant changes noted. This necessitates the need for further research addressing this concern to provide more evidence-based findings for practice. Results of another systematic review conducted among paediatric patients concluded that pulmonary vasodilators lower mortality rates in paediatric pulmonary hypertension patients, improve respiratory and hemodynamic parameters, and minimize the period of mechanical ventilation (27). While findings of another study from Kimuro et al. concluded that pulmonary vasodilators for pulmonary hypertension in haemodialysis patients with chronic kidney disease reduce pulmonary vascular resistance and, ultimately, increase exercise tolerance. Pulmonary vasodilators may benefit haemodialysis patients with pre-capillary pulmonary hypertension, but they must be managed carefully due to the peril of pulmonary edema (28).
In pediatric populations, the use of vasodilators such as albuterol has been associated with an increased risk of emergency room visits. Albuterol, a beta-agonist bronchodilator commonly used to relieve bronchospasm in children and adolescents with asthma or other respiratory conditions, can induce systemic vasodilation as a side effect. This vasodilatory effect may lead to adverse outcomes such as tachycardia, hypertension, and exacerbation of underlying conditions, potentially necessitating emergency medical attention (29). Similarly, in this review, one of the included studies reported the use of bronchodilators; however, 6.7% of children and 9.3% of adolescents exhibited poor bronchodilator dose responsiveness, and the use of 720 mcg albuterol, as per the findings of this study, was linked to a 2-fold increase in emergency department visits and a 3-fold increase in hospitalizations after 12 months. Hall et al. concluded that emergency department direct dispensing of beta-agonist metered dose inhalers resulted in decreased 28-day visits and hospital readmissions (30).
This systematic review offers valuable insights into the utilization of pulmonary vasodilators in acute pulmonary conditions, but it also comes with its own set of strengths and limitations. One of the key strengths of this systematic review is its comprehensive approach to synthesizing the existing literature on pulmonary vasodilators in emergency medicine. By systematically searching multiple databases and employing rigorous inclusion criteria, the review ensures a thorough coverage of relevant studies, thereby enhancing the reliability and generalizability of its findings. Additionally, the systematic review likely involved a thorough quality assessment of included studies, allowing for a critical appraisal of the evidence and minimizing the risk of bias in the interpretation of results. Furthermore, the review's focus on emergency medicine provides timely and practical insights for clinicians managing acute pulmonary conditions in high-stakes settings. By specifically addressing the role of pulmonary vasodilators in emergency care, the review addresses a critical knowledge gap and offers guidance on evidence-based treatment strategies in this context. This targeted approach enhances the relevance and applicability of the review's findings to frontline emergency medicine practitioners, potentially informing clinical decision-making and improving patient outcomes.
However, like any systematic review, there are also limitations to consider. One potential limitation is the inherent heterogeneity of the included studies in terms of patient populations, interventions, and outcomes assessed. Variability in study designs and methodologies may introduce challenges in data synthesis and limit the ability to draw definitive conclusions. Additionally, heterogeneity observed in the results or study outcome measures may further limit the generalizability of the findings; however, this heterogeneity may have resulted due to the intrinsic characteristics of the studies. Moreover, the review's reliance on existing literature means that it is inherently limited by the quality and quantity of available evidence. Insufficient data or conflicting results in the literature may restrict the review's ability to provide definitive recommendations or insights into certain aspects of pulmonary vasodilator therapy in emergency medicine.
Future directions
Future research in the field of pulmonary vasodilators in emergency medicine should focus on addressing key knowledge gaps and advancing our understanding of optimal treatment strategies in acute pulmonary conditions. One important direction for future research is the investigation of personalized approaches to pulmonary vasodilator therapy, taking into account individual patient characteristics, disease severity and underlying pathophysiology. By identifying biomarkers or clinical predictors of treatment response, clinicians can better tailor vasodilator therapy to the specific needs of each patient, optimizing efficacy and minimizing adverse effects. Additionally, there is a need for further research into alternative delivery methods and formulations of pulmonary vasodilators to enhance their practicality and accessibility in emergency settings. Novel approaches such as inhaled prostacyclin analogs, sustained-release formulations, or combination therapies may offer advantages in terms of ease of administration, duration of action, and patient tolerance, potentially improving treatment outcomes and reducing healthcare resource utilization. Furthermore, future studies should explore the long-term effects and outcomes of pulmonary vasodilator therapy in emergency medicine. While much of the existing literature focuses on short-term outcomes such as oxygenation and hemodynamic stability, there is a paucity of data on the impact of vasodilator therapy on patient-centered outcomes such as mortality, quality of life, and healthcare utilization beyond the acute setting. Longitudinal studies and prospective trials are needed to assess the durability of treatment effects, the risk of rebound pulmonary hypertension, and the overall impact on patient outcomes over time. Moreover, given the evolving landscape of acute pulmonary conditions and emerging therapeutic modalities, future research should also investigate the role of pulmonary vasodilators in conjunction with other treatment modalities such as thrombolytics, immunomodulators, and mechanical support devices. Collaborative research efforts and interdisciplinary approaches will be essential to advance our understanding of pulmonary vasodilator therapy and optimize its integration into comprehensive management strategies for acute pulmonary emergencies.
Conclusion
The review highlights the significant impact of pulmonary vasodilators in improving oxygenation, reducing pulmonary vascular resistance, and alleviating symptoms associated with conditions such as acute pulmonary embolism and pulmonary arterial hypertension, with a minimum number of studies also indicating no significant differences or improvements with the initiation of vasodilator therapy, which necessitates the need for further research to provide more evidence-based findings. Future studies should focus on personalized approaches to therapy, alternative delivery methods, and the impact of pulmonary vasodilators on patient-centred outcomes, ultimately informing evidence-based practice and enhancing patient care in emergency medicine settings.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection, and final writing of the manuscript.