Volume 4, Issue 8
August 2024
Effectiveness of Integrating Palliative Care into Ambulatory Care of Non-cancer Terminally Ill Patients; A Systematic Review
Saeed Yahya Alzahrani, Hassan Yousef Alghar, Anwar Mohammed Alshehri, Maied Zaher Alshehry
DOI: http://dx.doi.org/10.52533/JOHS.2024.40802
Keywords: outcome, palliative, care, ambulatory, quality, life
With the increase in the burden of chronic diseases, the need for and access to better healthcare is required to improve the quality of life of patients. Palliative care is an interdisciplinary specialization that aims to enhance the quality of life for individuals with serious life-limiting illnesses, regardless of their stage of disease, as well as caregivers. Integration of early palliative care into an ambulatory care setup can help improve the overall care experience for patients and their families while also addressing their unique physical, emotional, and psychosocial needs, and it ensures continuity of care throughout a patient's disease trajectory. We aim to conduct this systematic review to assess the impact of the incorporation of early palliative care for terminally ill non-cancer patients and describe the effectiveness of palliative care in terms of symptom control and management and overall quality of life. We have conducted both electronic and manual search strategies within the potential databases and included articles to find relevant studies. Studies from 2013-2023 were included. Original studies that recruited non-cancer terminally ill patients receiving palliative care in ambulatory care settings were included, while studies including pediatric patients (age less than 18 years) were excluded. Cochrane bias risk assessment tool was utilized to assess the quality of the studies. The final inclusion resulted in 10 studies. Integration of palliative care in ambulatory care settings improved the quality of life among patients and decreased their symptom burden and hospitalization rate. Few studies also reported improved survival times among their patient populations. Mean changes from baseline symptoms were reported. Integration of palliative care in ambulatory care shows promising results. Although to further improve patient-centered outcomes and consider patients' perspectives on care delivery, more research is necessary to develop an empirical understanding of palliative care by recognizing and addressing the characteristics and implementation issues crucial to integrating these models in ambulatory care.
Introduction
Chronic non-cancer diseases like dementia, heart failure, and chronic obstructive pulmonary disease, among certain others, are prevalent and linked to increased healthcare utilization, a high burden of symptoms, disability, and a lower quality of life. Palliative care is centered on enhancing the quality of life, lessening suffering, and supporting patients and caregivers in making decisions. Palliative care benefits are currently mostly demonstrated for cancer patients. However, the number of people requiring palliative care due to non-cancer diseases is twice that of patients with cancer (1). Individuals with a high burden of symptoms who are terminally ill are entitled to specialized palliative care. When there is no longer hope for recovery, the goal of palliative care is to lessen the effects of an illness. Palliative care has become more widely used in the treatment of various progressive chronic diseases despite its roots in cancer medicine (2).
Decades ago, palliative care was developed with the goal of improving the quality of life for patients who are terminally ill. Palliative care for patients other than cancer patients has taken on more importance because of the developed world's growing aging population. Additionally, palliative care can improve the course of dementia, chronic obstructive pulmonary disease, and congestive heart failure. However, challenges for caregivers of terminally ill non-cancer patients include developing and modifying care plans over extended periods, initiating advance care planning at a later time, and managing symptoms in concert with many physicians. Both cancer and non-cancer patients can overcome their disease burdens with palliative care. Palliative care can alleviate symptoms associated with nearing death, alleviate psychological anguish, and enhance quality of life. Increased do-not-resuscitate consent, improved breathlessness, and improved diagnosis recognition by patients and families can all be achieved by the early incorporation of palliative care (3).
Patients with serious chronic non-cancer diseases receive the majority of their care in ambulatory settings, especially in physician's offices. Due to their frequent high symptom burden and reduced quality of life in relation to their health, people with chronic diseases might require specialized care. Increased palliative care integration into ambulatory care, whether through the addition of palliative care services or the training of ambulatory care professionals in palliative care, may be advantageous to these patients (4). Moreover, palliative care provided in clinics for people with terminal illnesses has great potential. Evidence from research studies has demonstrated that ambulatory palliative care clinics can result in increased survival rates, decreased use of medical services, and enhanced quality of life (5). Reducing physical pain as well as the psychological, spiritual, and emotional anguish associated with a life-limiting illness is the ultimate objective of palliative care. Despite being a human right, access to palliative care varies greatly; of the 40 million people who require it worldwide, less than 10% obtain it, mostly in high-income nations (6).
Furthermore, discussions about end-of-life care and measures to manage severe symptoms typically take place in the final few weeks of life. This tardiness is frequently linked to care that is mostly provided in hospital environments. Early in a patient's illness and even in conjunction with active treatments, palliative care can significantly enhance symptom control and lessen the discomfort imposed by conventional therapy. Previous clinical trials have demonstrated that earlier palliative care is linked to better outcomes for patients towards the end of their lives. According to recent studies, community-based palliative care may also result in advantages, including fewer hospital deaths and acute hospitalizations (7). The quality of life and general health of patients can be significantly improved by the early inclusion of palliative care. It gives patients the assistance they need to control their symptoms, lessen their pain, and enhance their mental and physical health. Additionally, it assists patients and their families in making future decisions by helping them comprehend their condition better. Palliative care should not be postponed until later in the course of therapy, since this may lead to lost opportunities and a less thorough approach to patient care (8).
Integration of early palliative care into an ambulatory care setup can help improve the overall care experience for patients and their families while also addressing their unique physical, emotional, and psychosocial needs, and it ensures continuity of care throughout a patient's disease trajectory. Moreover, early palliative care ensures that end-of-life care planning and discussions are integrated into the patient's care from an early stage. This can help patients and families make decisions that align with their wishes and values. However, studies in the literature focus more on palliative care for cancer patients, and to highlight the importance and present evidence-based findings, we aim to conduct this systematic review to assess the impact of the incorporation of early palliative care for terminally ill non-cancer patients and describe the effectiveness of palliative care in terms of symptom control and management and overall quality of life.
Methods
Definition of Outcomes and Inclusion Criteria
Our study aimed to determine the impact and effectiveness of palliative care in an ambulatory care setting. As a result, we included original studies that recruited non-cancer terminally ill patients receiving palliative care. Studies including pediatric patients (aged less than 18 years) were excluded. Moreover, case reports with limited sample sizes and no descriptive statistics were also excluded from this review. Other exclusion criteria were nonhuman or laboratory studies, non-original investigations or incomplete studies, abstract-only articles, protocols, theses, and articles that were not published in English or with no available English information. A total of 10 studies published from 2013-2023 were included.
Search Strategy
Following the successful completion of our intended results, we performed a brief manual screening of potentially included studies to identify relevant keywords for the most appropriate search term. Our search terms included (Non-cancer terminally ill patients OR actively dying OR end of life OR terminally ill OR terminal care OR transition of care) AND (Integrated palliative care OR Palliative care patients OR Palliative medicine OR Palliative treatment OR Palliative therapy OR Palliative care Unit OR Palliative Care Nursing) AND (Outpatient OR clinic visit OR ambulatory care OR health Service OR urgent care) AND (symptom management OR patient comfort OR quality of life). The databases employed for searching included PubMed, Web of Science and Cochrane Library. To ensure the inclusion of all relevant research studies, our search was restricted to the title and abstract of the search results. Once all of our results had been transferred and saved to an Endnote library, we traversed each database to find and eliminate duplicates. Furthermore, we conducted a manual search of the included studies' reference lists, related reviews, and comparable article sections in PubMed to find any papers that our electronic search technique may have overlooked. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines throughout all stages of this systematic review.
Screening and extraction
To ensure the accuracy and quality of our review process, we implemented a double screening strategy, which involved screening both titles/abstracts and full texts. Two reviewers conducted the screening process in a blind manner, and a senior member overlooked the entire process and facilitated discussions among the reviewers in case of discrepancies. We constructed an extraction sheet that was organized in a manner relevant to our research objectives, which included baseline characteristics, publication details, abstracts, decisions to include or exclude articles, and the reasons for exclusion. We also identified whether each study was a clinical trial or not. We made sure to include all relevant articles that met our criteria.
Quality Assessment
We extracted information from the included studies regarding the potential risk of bias in these studies. To assess the quality of randomized control trial studies, we used the Cochrane bias risk assessment tool. It consists of six domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting. The tool also assesses others and the overall risk of bias.
Results
Search Results
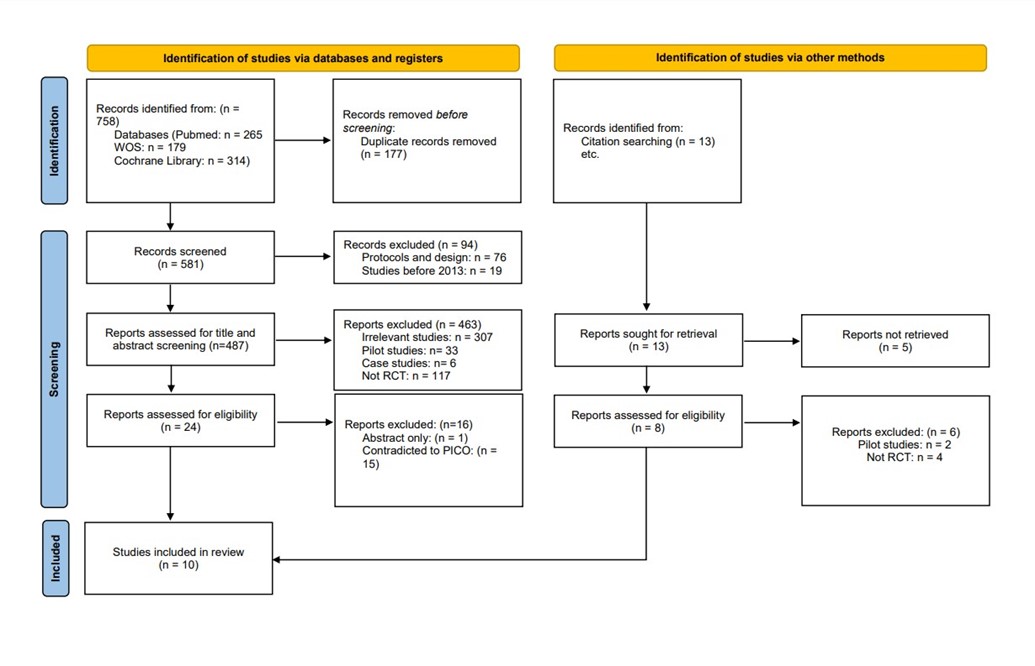
We conducted the search strategies as described above and identified a total of 581 citations, which were then reduced to 487 after removing duplicates. After screening titles and abstracts, only 24 citations were considered eligible for the next steps. Full-text screening narrowed down the number to 10 articles that matched our inclusion and exclusion criteria. (Figure 1) (9) shows the detailed search strategy and screening process.
Results of Quality Assessment
The quality assessment of the included studies revealed that overall, the majority of studies had a high risk of bias, while only two of the included studies exhibited a low risk of bias. The detailed results of the quality assessment are illustrated in (Table 1).
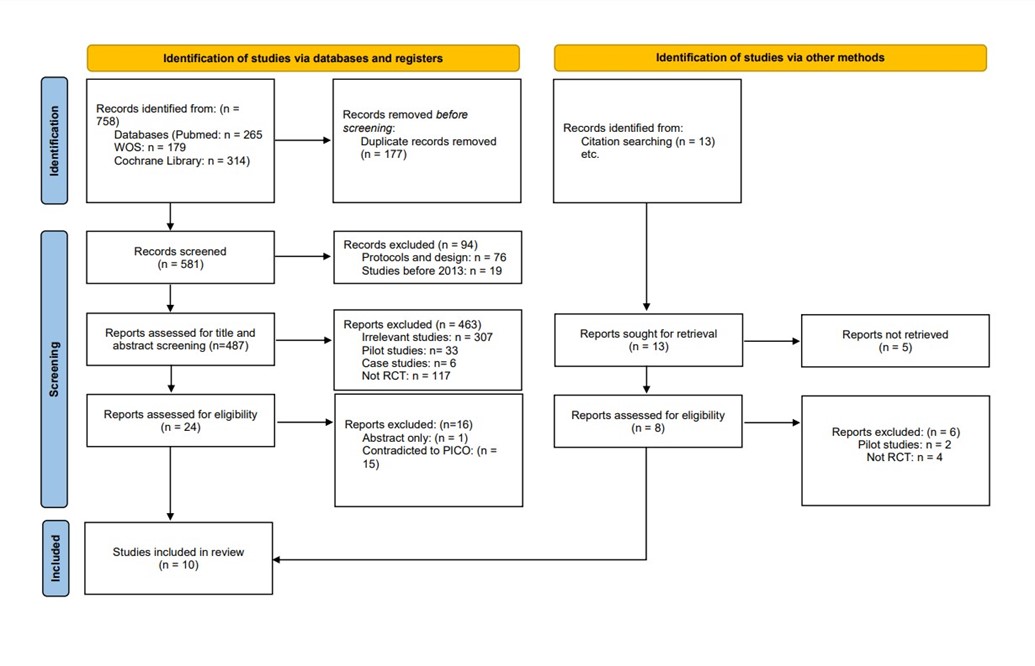
Figure 1: PRISMA Diagram
|
Table 1: Quality assessment of included studies using Cochrane bias risk. |
||||||||
|
Studies |
Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective reporting |
Other bias |
Overall |
|
Bekelman et al (9) |
Low |
Low |
High |
High |
High |
Low |
High |
High |
|
Dionne Odom et al (10) |
Low |
Unclear |
High |
Low |
High |
Low |
Low |
High |
|
Evans et al (11) |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Higginson et al (12) |
Low |
Low |
Low |
Low |
High |
Unclear |
Low |
Low |
|
Kluger et al (13) |
Unclear |
Unclear |
High |
Unclear |
Low |
Low |
High |
High |
|
W. Ng et al (14) |
Low |
Low |
High |
Low |
Low |
Low |
High |
High |
|
Nkhoma J et al (15) |
Unclear |
Low |
Low |
Low |
High |
High |
High |
High |
|
Sidebottom et al (16) |
Unclear |
High |
High |
Unclear |
High |
High |
High |
High |
|
Rogers JG et al. (17) |
Unclear |
Unclear |
High |
High |
Low |
Low |
Low |
High |
|
Bekelman DB et al. (18) |
Low |
Low |
High |
Unclear |
High |
Low |
Low |
High |
Characteristics of the included studies
We included 10 studies that recruited 2779 patients and were published between 2013 and 2020 (10-19). Among the case groups total population, there were 583 males and 350 females, while the control group population was comprised of 449 males and 313 females. All of the included studies were randomized controlled trials. Regarding the geographical distribution of the included studies, the majority were from the United States, followed by England, China, and Malawi. All the baseline characteristics of these studies are shown in (Table 2). There are variations in the sample size of included papers, which are likely due to the objective and inclusion criteria of the specific study.
Study outcome measures
In the study's outcome measures, the majority of the study population comprised heart failure patients, while others suffered from Parkinson's disease, HIV/AIDS, and chronic non-cancer diseases. The authors employed diverse assessment scales and tools to evaluate the impact of intervention, specifically the integration of palliative care, on symptom management and control, with mean changes reported. For the assessment of depression and anxiety, the Hospital Anxiety and Depression Scale (HADS) was used. Dionne Odom et al. observed a 3.9± 3.1/3.7±2.9 score for anxiety and 4.7±3.1/4.8±3.3 for depression (11). Similarly, Rogers et al. reported quite closer scores for anxiety (3.7±4.0/6.2±4.8 and 4.6±3.6/6.4±4.3 among patients with heart failure), (18) while Higginson et al. noted comparatively increased scores 9.2±2.8/9.1±2.7 for anxiety and 10.0±2.8/11±2.5 for depression (13). Kluger et al. reported a mean change of −0.33 (−0.92 to 0.25) for depression at 12 months and 0.12 (−0.45 to 0.69) for anxiety (14). Bekelman et al. observed a mean change of -2.2/-0.8 for Patient Health Questionnaire-9 (PHQ-9) at 6 months, while Sidebottom et al. reported a change of 14.86/11.80 after 3 months (10, 17). In Bekelman et al.'s 6-month intervention for chronic heart failure patients, participants experienced notable improvements in various health outcomes. Specifically, the mean Kansas City Cardiomyopathy Questionnaire (KCCQ) score increased by 5.5 points in the intervention group, indicating a positive impact. The difference in mean scores between the two groups was 2.6 points, with a 95% confidence interval of –1.3 to 6.6 (10).
|
Table 2: Baseline characteristics of the included studies |
|||||||||||||
|
No. |
Author |
Registration |
Country |
Study type |
Year |
Study period |
Total no. |
Cases no. |
Control no. |
Age (Years) (cases/control) Mean |
Gender (M/F) cases |
Gender (M/F) control |
|
|
1 |
Bekelman et al (9) |
NCT01739686 |
USA |
RCT |
2018 |
2012- 2015 |
314 |
157 |
157 |
64.5±10.9/66.5±11.8 |
128/29 |
119/38 |
|
|
2 |
Dionne Odom et al (10) |
NCT02505425 |
England |
RCT |
2020 |
2015-2018 |
371 |
82 |
76 |
58.2±12.4/57.6±10.8 |
9/73 |
14/62 |
|
|
3 |
Evans et al (11) |
ISRCTN45837097 |
England |
RCT |
2021 |
NR |
50 |
24 |
26 |
85.3±6.4/86.0±5.7 |
12/12 |
14/12 |
|
|
4 |
Higginson et al (12) |
NCT01165034 |
England |
RCT |
2014 |
2010-2012 |
105 |
53 |
52 |
66±11/68±11 |
28/25 |
33/19 |
|
|
5 |
Kluger et al (13) |
NCT02533921 |
USA |
RCT |
2020 |
2015- 2017 |
584 |
104 |
106 |
69.5±8.3/70.7±8/ |
70/34 |
65/41 |
|
|
6 |
W.Ng et al (14) |
NR |
China |
RCT |
2018 |
2013-2015 |
84 |
43 |
41 |
78.3±16 /78.4±10 |
18/25 |
25/16 |
|
|
7 |
Nkhoma J et al (15) |
NR |
Malawi |
RCT |
2015 |
2012-2013 |
182 |
92 |
90 |
40.5±11.3/41.3±11.65 |
43/49 |
56/34 |
|
|
8 |
Sidebottom et al (16) |
NR |
USA |
RCT |
2015 |
2012-2013 |
547 |
116 |
116 |
76±11.9/70.9±13.6 |
55/61 |
67/49 |
|
|
9 |
Rogers et al (17) |
NCT01589601 |
USA |
RCT |
2017 |
2012-2015 |
150 |
75 |
75 |
71.9±12.4/69.8±13.4 |
42/33 |
37/38 |
|
|
10 |
Bekelman et al (18) |
NCT00461513 |
USA |
RCT |
2015 |
2009-2011. |
392 |
187 |
197 |
67.3±9.6/67.9±10.6 |
178/9 |
193/4 |
|
RCT: Randomised controlled trial; NR: Not reported
In a separate study by Rogers et al., patients receiving usual care along with palliative care (UC+PAL) demonstrated substantial enhancements in the KCCQ overall summary score over 6 months compared to those receiving usual care alone. The improvement was quantified as a 9.49-point difference, with a 95% confidence interval ranging from 0.94 to 18.05, underscoring the significant positive impact of incorporating palliative care into the standard care regimen (18). Significant improvements were also observed in the Edmonton Symptom Assessment System (ESAS), with Kluger et al. reporting a substantial mean change at 12 months for Parkinson's Disease patients, indicating a marked reduction in symptom burden (14).
Ng et al.'s 12-week intervention resulted in a statistically significant reduction in the median total score on the ESAS. In Sidebottom et al.'s study, the 3-month change in ESAS Total Score highlighted a substantial decrease in the intervention group compared to the control group. The mean difference between groups was statistically significant at 4.31, emphasizing the intervention's significant impact on alleviating the overall symptom burden. The mean change in the intervention group over 3 months was 3.69, further highlighting positive outcomes across diverse symptom domains (15, 17).
In Evans et al.'s study, the assessment of carer burden using the Zarit-12 items at 12 weeks revealed no statistically significant difference between the intervention and control groups (12). This suggests that the palliative care intervention did not have a significant impact on carer burden compared to standard care in the specified time frame. On the other hand, Kluger et al.'s investigation at 12 months showed a significant reduction in caregiver burden among Parkinson's disease patients in the palliative care intervention group compared to standard care. The intervention group exhibited a substantial mean change, while the standard care group had a minimal mean change. The estimated difference between groups was -2.60, indicating that the palliative care intervention led to a notable reduction in caregiver burden, as measured by the Zarit Burden Interview, after 12 months. In Ng et al.'s study, the palliative care intervention also demonstrated a significant reduction in caregiver burden, supporting the effectiveness of the intervention across multiple studies (12, 14, 15).
While, according to the Quality of Life/Quality of Life in Alzheimer's Disease scale, Ng et al. noted a mean of 7.87/6.84, Kluger et al. observed a change of 0.68/−0.43 after 12 months in their study (14, 15). For the Patient-Reported Outcomes Measurement Information System (PROMIS), Bekelman et al. observed a mean change of -2.8/-0.8 at 6 months, while Dionne Odom et al. reported scores for physical assessment and mental assessment (10, 11).
|
Table 3 (A): Outcome measures of the included studies |
||||||||||
|
Srno |
Author |
Diagnosis |
Group |
Depression & Anxiety HADS |
PHQ-9 |
KCCQ |
ESAS |
ZBI |
QOL/QOL-AD |
PROMIS (global health) |
|
1 |
Bekelman et al (9) |
Chronic HF |
Intervention/Usual care |
NR |
Mean change after 6 months -2.2/-0.8, Difference Between Change Scores (95% CI): −1.4 (−2.6 to −0.2) |
Mean change after 6 months 5.5/2.9, Difference Between Change Scores (95% CI): :2.6 (−1.3 to 6.6) |
NR |
NR |
NR |
At 6 months (mean changes -2.8/-0.8 Difference Between Change Scores (95% CI): -2.0; -3.6 to -0.4; |
|
2 |
Dionne Odom et al (10) |
HF |
Intervention/Usual care |
Anxiety :3.9± 3.1/ 3.7 ±2.9; Depression: 4.7 ±3.1 /4.8 ±3.3 |
NR |
NR |
NR |
NR |
NR |
Physical 46.9±8.9/48±8.6 Mental 48.5±7.1/48.1±7.9 |
|
3 |
Evans et al (11) |
Chronic non-cancer diseases |
Intervention/Usual care |
NR |
NR |
NR |
NR |
3.56±5.68/2.23±6.30; Mean difference: -1.80(-2.98 to 6.57) |
NR |
NR |
|
4 |
Higginson et al (12) |
Breathlessness |
Intervention/Usual care |
Anxiety: 9.2±2.8/9.1±2.7 Depression: 10.0±2.8/ 11±2.5 |
NR |
NR |
NR |
NR |
NR |
NR |
|
5 |
Kluger et al (13) |
Parkinsons disease |
Intervention/Usual care |
Mean of change after 12 months Depression: −0.33 (−0.92 to 0.25)/ 0.12 (−0.45 to 0.69) Anxiety: 0.12 (−0.45 to 0.69)/ −1.42 (−2.04 to −0.80) |
NR |
NR |
Mean of change after 12 months: −9.66 (−13.52 to −5.80)/ −0.73 (−4.97 to 3.51) |
Mean Change after 12 months: −2.25 (−3.56 to −0.94)/−0.02 (−1.32 to 1.37) |
Baseline 33.9±5.7/34.3±5.6 Change after 12 months: 0.68 (−0.38 to 0.73)/ −0.43 (−1.37 to 0.50) |
NR |
|
6 |
W Ng et al (14) |
HF |
Intervention/Usual care |
NR |
NR |
NR |
Median (IQR): 2.11(0.78-3.22)/2.22(0.94-3.42) |
Median (IQR): 11.61(7.3-18.83)/23(12.06-36) |
Mean (95% CI) 7.87(7.35-8.39)/ 6.84(6.28-7.40) |
NR |
|
7 |
Nkhoma J et al (15) |
HIV/AIDS |
Intervention/Usual care |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
|
8 |
Sidebottom et al (16) |
HF |
Intervention/Usual care |
NR |
Change after 3 months 14.86/ 11.80 |
NR |
Change after 3 months 11.00/6.70 |
NR |
NR |
NR |
|
9 |
Rogers et al (17) |
HF |
Intervention/Usual care |
Depression: 4.6±3.6/6.4±4.3 Anxiety: 3.7±4.0/6.2±4.8 |
NR |
26.3±19.42/22.2±24.69 |
NR |
NR |
NR |
NR |
|
10 |
Bekelman et al (18) |
HF |
Intervention/Usual care |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
HADS: Hospital Anxiety and Depression Scale; PHQ-9: Patient Health Questionnaire-9; KCCQ: Kansas City Cardiomyopathy Questionnaire; ESAS: Edmonton Symptom Assessment System; ZBI: Zarit Burden Interview; QOL/QOL-AD: Quality of Life/Quality of Life in Alzheimer's Disease; PROMIS: Patient-Reported Outcomes Measurement Information System; NR: Not reported; CI: Confidence interval; HF: Heart failure.
Nkhoma et al. reported that although both groups had reduced average pain severity at follow-up, patients in the intervention group experienced a greater decrease in pain severity (16). Bekelman et al. reported that among the collaborated care or interventional group, there were 18 patients with 1 hospitalization and 9 with ≥2 hospitalizations, while in the usual care group, there were 30 patients with 1 hospitalization and 6 with ≥2 hospitalizations. Moreover, depression symptoms improved in the collaborative care group, and this change persisted after 12 months; however, fatigue symptoms did not persist (10).
Higginson et al. reported a 0.8±3.6/1.3±4.3 hospitalization rate, and the survival rate from randomization to 6 months was better in the breathlessness support service group than in the control group (13). Although Bekelman et al. observed no significant difference in 1-year hospitalization rates between the intervention arm and the usual care arm, there were significantly fewer deaths in 1 year in the intervention arm (19). Sidebottom et al. reported that palliative care may improve symptom burden, depressive symptoms, and quality of life in heart failure patients, and those in the intervention group were 2.87 times more likely to have completed the disease-specific ACP process within 6 months (17). Both the outcome measure and clinical outcomes of these studies are shown (Table 3A) and (Table 3B). Overall, the results suggest that the integration of palliative care in an ambulatory care setting or outpatient improved the quality of life among patients, decreased their symptom burden and hospitalization rate, and, in some studies, observed better survival in the group that received palliative care.
|
Table 3 (B): Clinical outcomes of the included studies |
||||
|
SR. No |
Author |
Hospitalization rates |
Survival outcome |
Symptom management/Symptom reduction |
|
1 |
Bekelman et al (9) |
CASA, 18 patients with 1 hospitalization and 9 with ≥2 hospitalizations; usual care, 30 patients with 1 hospitalization and 6 with ≥2 hospitalizations; P = .61 |
NR |
CASA at 6 months: Depressive symptoms improved with CASA (the effect size was –0.29 at 6 months [P = .02]). This effect persisted at 12 months (effect size, –0.36; P = .006). Fatigue improved by (effect size, –0.30; P = .02), yet this change did not persist at 12 months (effect size, –0.18; P = .16) |
|
2 |
Dionne Odom et al (10) |
NR |
NR |
NR |
|
3 |
Evans et al (11) |
NR |
NR |
NR |
|
4 |
Higginson et al (12) |
0.8±3.6/1.3±4.3 |
Survival rate from randomization to 6 months was better in the breathlessness support service group than in the control group (50 of 53 [94%] vs 39 of 52 [75%]) and in overall survival (generalized Wilcoxon 3·90, p=0?048). |
NR |
|
5 |
Kluger et al (13) |
NR |
NR |
NR |
|
6 |
W Ng et al (14) |
NR |
NR |
NR |
|
7 |
Nkhoma J et al (15) |
NR |
Both groups had reduced average pain severity at follow-up. However, those in the pain education group had a mean change of 40.95 (SD = 23.78), whereas the usual care group had a mean change of 19.27(SD = 25.27) |
Patients in the intervention group experienced a greater decrease in pain severity (mean difference = 21.09 points, 95% confidence interval = 16.56-25.63; P < 0.001). |
|
8 |
Sidebottom et al (16) |
Inpatient and emergency department admissions and total inpatient length of stay in the 6 months prior to the study were compared as proxy measures for disease severity, but no differences were found between the two groups for these measures |
Those in the intervention group were 2.87 times more likely to have completed the disease-specific ACP process within 6 months |
PC may improve symptom burden, depressive symptoms, and QOL in HF patients |
|
9 |
Rogers et al (17) |
patients had an average of 2.2 hospitalizations in the 12 months prior to enrolment |
NR |
NR |
|
10 |
Bekelman et al (18) |
no significant difference in 1-year hospitalization rates between the intervention arm and the usual care arm (29.4% vs 29.9%, P = .87). |
significantly fewer deaths at 1 year in the intervention arm (8 of 187 [4.3%]) than in the usual care arm (19 of 197 [9.6%]) (P = .04) |
- |
NR: Not reported; QOL: Quality of life; PC; Palliative care; HF; Heart failure; CASA: Collaborative Care to Alleviate Symptoms and Adjust to Illness; ACP: advance care planning; PD: Parkinson Disease
Discussion
This study aimed to determine the impact or influence of early integration of palliative care in ambulatory care settings for non-cancer terminally ill patients. The findings of this study signify that early palliative care is beneficial for this vulnerable group of patients, as results showed improvement in symptoms over time, which leads to a better quality of life. Few studies also reported improved survival time among their patient population.
In this review, the majority of the study population comprised heart failure patients, and palliative care seemed to play a vital role in the management of symptoms and decreasing hospitalization rates. Similarly, results of another review described that for patients with heart failure, outpatient palliative care reduced symptoms, enhanced quality of life, and reduced the need for rehospitalization. By addressing psychosocial, emotional, and spiritual requirements, care coordination, and family engagement in care, integrating palliative care improves traditional heart failure management. The authors further suggested that early palliative care integration in non-hospital settings is crucial because of the chronic and unpredictable nature of heart failure (20). Results of another systematic review also demonstrated that a palliative approach is linked to better results for caregivers, decreased symptom load and burden, and an improvement in patient quality of life (21). Corresponding with these further findings of another review also affirmed the importance of palliative care measures for patients with chronic heart failure and the people who care for them in terms of a variety of outcomes, especially psychological and quality of life (22). Likewise, three studies in our review showed improved mean scores for anxiety and depression among patients receiving palliative care.
Results of a study by Gaddoud et al. showed that, compared to 48% of cancer patients, just 7% of individuals with heart failure were listed on the palliative care registry and 29% of heart failure patients listed on the palliative care registry, were added to the list within seven days of their death. This validates the glaring disparity in the identification of palliative care needs among individuals with heart failure throughout a significant primary care population. Hence, the authors further recommended shifting the criteria for palliative care from ones based on prognosis to ones that are patient-centered, assessing and attending to palliative needs along the process, including advance care planning (23). Similarly, Kavalieratos stated that although there is a growing body of evidence in support of palliative care for heart failure patients, this field is still in its infancy and will require more outstanding methodologically sound research to elaborately understand the role of palliative care for heart failure patients and their families. However, more focus on primary palliative care, such as advance care planning, and basic physical and emotional symptom treatment delivered by cardiology and primary care providers may be a means of addressing unmet palliative requirements early in the course of the illness (24).
Research evaluating individuals with a spectrum of diseases, such as stroke, heart disease, end-stage lung disease, renal failure, Parkinson's disease, and Alzheimer's disease, has clearly shown the unmet palliative care needs of patients dying from non-malignant disease. Findings of a survey in the United Kingdom also revealed that a significant proportion of general practitioners agreed that patients with non-malignant diseases like stroke, cardiovascular disease, chronic obstructive pulmonary disease, multiple sclerosis, and rheumatoid arthritis should be admitted to palliative care units. Nevertheless, very few patients with non-malignant diseases receive a referral for specialized palliative care, and even those facilities that are willing to take these patients report very low demand (25).
Mounsey et al. described that the primary objective of palliative care is to enhance a patient's quality of life when they have terminal conditions. Although it is frequently associated with cancer care, patients with end-stage non-cancerous diseases also have substantial needs. Similar to individuals with advanced cancer, patients with end-stage nonmalignant disease have comparable symptom burdens and care requirements. Palliative care, which includes treating the underlying illness and attending to symptoms, psychosocial needs, and caregiver support, is beneficial for these individuals. The option to plan for future disease episodes, including the provision of end-of-life care, is presented by advance care planning (26).
As part of universal health coverage, the World Health Organization (WHO) declared that all patients with serious illnesses and their families should receive palliative care. Furthermore, according to the WHO, non-cancer patients make up the majority of patients in need of palliative care; nevertheless, non-cancer patients and their families have just lately been able to get palliative care. In accordance with the findings of previous research studies, people without cancer had noticeably greater psychological and physical symptoms than those with the disease (27). Liberman et al. described that although implementing palliative care services in an ambulatory primary care setting presents logistical challenges, doing so enhances physicians’ comfort and understanding of palliative care while also increasing access to palliative care for children with complicated chronic medical illnesses. Palliative care services and advanced care planning should be introduced early to children with complicated chronic medical problems to promote quality of life and medical decision-making, as well as to manage symptoms (28). However, for this review, we excluded the pediatric population and assessed the outcomes among adults and the elderly population. However, it is significant to note that early incorporation of palliative care is equally important among children as well.
The findings of our review further showed that among patients with Parkinson's disease and HIV/AIDS, better quality of life and symptom control were observed in those who received palliative care. Similarly, Katz et al. reported that palliative care has been shown to significantly improve the quality of life of both Parkinson's' spectrum disorder patients and their caregivers, according to the emerging evidence (29). Additionally, Lum HD et al. defined that patients' and family caregivers' quality of life may be enhanced by palliative care integration. Through continuous goals-of-care talks, assessment, and management of an extensive spectrum of physical, emotional, social, and spiritual needs, caregiver attention, and appropriate referrals to hospice, primary palliative care can be included in Parkinson's disease care and management (30). Moreover, Boersma et al. concluded that palliative care can help meet the requirements of individuals with Parkinson's disease and their caregivers. To address these needs, multidisciplinary treatment was well-received by the caregivers (31).
Furthermore, Gilliams et al. stated that early palliative care provided in an outpatient setting is a potential strategy that may influence HIV care retention (32). Results of a study by Goodkin et al. reported that palliative care has been linked to both an increase in an HIV-positive person's quality of life and an improvement in their functional level in daily living activities (33). Onyeka described that palliative care is essential for managing the disease's symptoms, but it also attempts to improve the patient's quality of life by prolonging the patient's life. The psychosocial effects of HIV/AIDS, cancer, and other life-limiting diseases, however, may not always be recognized and addressed during the hospital stay. This can lead to a scenario that is more complicated than the illness itself (34).
While comparing our study findings to the cancer patient population in the ambulatory setting, studies in the literature show that, in comparison to controls, patients randomized to outpatient specialty palliative care exhibited an absolute 14% improvement in 1-year survival (56% vs. 42%, p <.001). Additionally, the advantage in survival was noted at 6, 9, 15, and 18 months, with a 4.56-month greater median survival period (14.55 vs. 9.99 months). Quality of life was improved in comparison to controls with outpatient specialty palliative care (g =.18, p <.001), for both physical and psychological indicators (35). Similarly, another study by Ferrell et al. reported that patients who received the interdisciplinary palliative care intervention showed significantly higher scores for quality of life, symptoms, spiritual well-being, and reduced psychological distress than those in the conventional care group at 12 weeks. Furthermore, there were noticeably more completed advance care directives and overall referrals for supportive care among the patients in the intervention group. Although patients in the earlier stages of the disease seemed to benefit more than those in Stage IV (36). Additionally, De Palma et al. demonstrated that palliative care cancer patients had much-reduced rates of all aggressive treatment indicators, including emergency department visits, hospital admissions, stays in intensive care units, major operative room procedures, and fewer in-hospital deaths (37).
Moreover, Hallman and Newton stated that reduced symptom burden, financial strain, and emergency room visits for symptom management were observed with the integration of palliative care into outpatient oncology clinics. In order to make sure that patients' preferences are followed, palliative care also makes it easier for patients and clinicians to communicate more frequently (38). Early referral of cancer patients to palliative medicine is supported by national guidelines and randomized controlled trials. Advance care planning, symptom management, symptom treatment, and support for family members and caregivers are all fundamentally aided by palliative medicine. Palliative medicine integration in oncology reduces healthcare costs while improving patient outcomes (39). In terms of improvement in quality of life, symptom burden, and survival time, our results for non-cancer terminally ill patients correspond with these findings. However, it's noteworthy that studies in the literature, while defining outcomes of palliative care, target more cancer populations, and for other advanced disease or terminally ill patients, studies are quite limited, especially in outpatient settings, which highlights the importance of our study, which is one of the very few available systematic reviews specifically involving non-cancer patients and describing outcomes in an ambulatory setting to the best of our knowledge. Additionally, our review includes findings from all randomized control trial studies, which is the strength of this study. Moreover, the systematic search methodology and the analysis of all keywords in this field add to the advantages and strengths of this study. Our study has certain limitations, including the inclusion of studies with a high risk of bias, possibly attributed to the intrinsic characteristics of the studies and the stringent criteria of the bias risk assessment tool employed. Secondly, all studies utilized diverse tools for symptom or outcome assessment, and due to this heterogeneity, the results may not be generalizable to the entire population of non-cancer patients. This underscores the necessity for further research that specifically includes non-cancer terminally ill patients in longitudinal and population-based studies using uniform criteria and tools. This approach will enable the generation of more evidence-based findings that can be generalized to the broader population.
Conclusion
Our results suggest that early integration of palliative care in an ambulatory setting can help improve the quality of life of non-cancer terminally ill patients. Additionally, it can also aid in the management of symptoms and improve survival time. However, in order to further improve patient-centered outcomes and take into account patients' perspectives on care delivery, more research is necessary to develop the empirical understanding of palliative care by recognizing and addressing characteristics and implementation issues crucial to integrating these models in ambulatory care.
Disclosure
Authors Contributions
All authors contributed equally to the making of this systemic review.
Declarations of Interest Statement
The authors declare no competing interest.
Data Availability
All data is provided within the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Statements of Ethics
Non-applicable.