Volume 4, Issue 4
April 2024
Hepatocellular Carcinoma (HCC): A Case of Ruptured Hepatocellular Carcinoma Associated with Liver Schistosomiasis
Eyad Tantawi, Fadi Albadawi, Abdulrahman Alharthi, Omar Althobity, Abdullah Alshanbari, Fares Alghamdi, Wied Qamrah, Ibrahim kharoub, Rana AL-Zaidi, Wael Alhoseny
DOI: http://dx.doi.org/10.52533/JOHS.2024.40403
Keywords: Abdominal hemorrhage, Liver, Mortality, Ruptured, Tumor, Schistosomiasis
Background: Hepatocellular carcinoma (HCC) makes up 75% of liver cancer cases and is recognized as the sixth most common cancer worldwide. Ruptured HCC can occur in 3 to 15% of cases, and it is the third most common cause of mortality in patients with HCC. Most patients are present with acute presentation of abdominal pain and signs of hemorrhagic shock. Nevertheless, the 5-year survival rate is between 5% and 15%, reflecting these patients' challenging management. We report a ruptured HCC, successfully treated with hepatic wedge resection.
Case presentation: A 56-year-old man from Yemen, who has no prior medical conditions, suddenly experienced severe abdominal pain after eating his dinner. Upon examination, he showed signs of generalized peritonitis, including rigidity, tenderness, and distention mainly in his right upper quadrant. An abdominal CT with IV contrast revealed fluid in his liver, right side abdomen, and pelvis, along with evidence of contrast blush at the extrahepatic portion of the hepatic lesion. A rupture of the lesion occurred, and a surgical intervention was performed to remove the bleeding liver segment. Afterwards, the patient recovered and was discharged for follow up.
Conclusion: Schistosomiasis is a significant parasitic infection that can lead to the development of HCC. It is important to monitor patients from regions where schistosomiasis is endemic to prevent this complication. The rupture of HCC is a critical event that requires immediate and aggressive management to avoid fatal outcomes. Additionally, the simultaneous treatment of both HCC and schistosomiasis is essential for optimal patient care.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver and a leading cause of cancer-related deaths worldwide (1). Moreover, HCC makes up 75% of liver cancer cases, and it has been reported to cause almost 800,000 new deaths annually (1-3). After tumor growth and liver failure, HCC rupture is the third most common cause of death from HCC, with a 25–75% mortality rate during the acute phase (4-6). However, the causes of rupture of HCC have been hypothesized to be rapid growth and necrosis, splitting of the surrounding parenchyma or vessel erosion, elevated intra-tumoral pressure with hepatic vein occlusion, and coagulopathy (4). Most cases of ruptured HCC present with abdominal pain, abdominal distention, and shock may occur in 33% of these cases (7, 8). For investigation, computed tomography (CT) imaging helps identify the presence of tumor bleeding, define the extent of the cancer, and display changes in the density of serial hematomas (9, 10). Immediate interventions for hemostasis have been reported to be the primary treatment for the rupture of HCC (7, 10). These interventions include emergency or staged hepatectomy (embolization or other conservative measures to achieve hemostasis followed by surgery), which can result in long-term survival (7, 10). Herein, we present a ruptured HCC caused by schistosomiasis infection with a massive abdominal hemorrhage managed by hepatic wedge resection.
Case presentation
We report a 56-year-old male patient from Yemen, not known to have any medical illnesses, presented to the emergency department with his family complaining of a first-time experience of sudden right upper quadrant pain that started 6 hours before presentation; the pain is acute and sharp, not radiated anywhere. It was then followed by a fainting attack and loss of consciousness. There was no nausea, vomiting hematemesis or history of hematochezia prior to the fainting attack. Moreover, there was no reported jaundice, fever, history of night sweats, weight loss or loss of appetite. The patient is not taking any medications or has known allergies. There are no previous surgeries or family history of malignancy.
On general examination, the patient was conscious, alert, and oriented. Initial vital signs showed temperature 37ºC, blood pressure 126/60 mmHg, pulse 103 beats/min, respiratory rate 18 breaths/min and oxygen saturation 98% on room air. An abdominal examination showed distention with rigidity and significant tenderness, mainly over the right upper quadrant, active bowel sounds and positive murphy signs while other clinical examinations were unremarkable.
Laboratory parameters results showed a white blood cells count of 11.27 k/uL, hemoglobin level of 13.1 g/dL, hematocrit of 30.8%, platelets of 549 (X10^9/L), creatinine of 56.7 umol/L, blood urea nitrogen 6 mmol/L, aspartate aminotransferase of 1094 U/L, alanine aminotransferase of 928 U/L, alkaline phosphatase of 109 U/L, lactate dehydrogenase 858 IU/L, total bilirubin of 15.3 umol/L, prothrombin time of 12.1 s, partial thromboplastin time of 25.7 s, international normalized ratio of 0.89 s, albumin of 40.8 g/dL, Venous blood gas showed Ph: (7.16), Pco2: (42), Hco3: (13.8), and Lactate: (8.2). Hepatitis C antibodies were positive, hepatitis B antibodies and surface antigen were negative, carcinoembryonic antigen (CEA) 1.23 ug/L, and carbohydrate antigen sialyl Lewis (CA 19-9) 1,4 U/mL. (post-operative result). Meanwhile, his abdominal and chest x-rays excluded any free intraperitoneal air and were unremarkable.
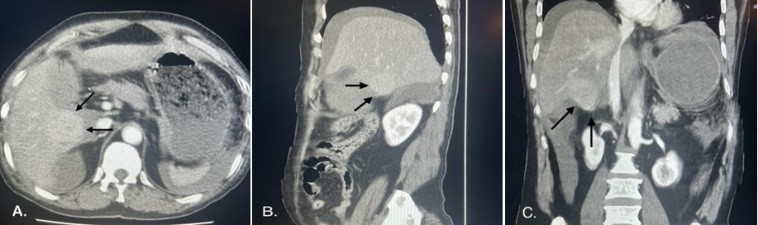
A contrast-enhanced computed tomography (CT) scan showed a well-circumscribed mass bulging exophytically from the inferior aspect of segment V of the right lobe in a mild cirrhotic liver with moderate ascites during the portal venous phase (Figure 1). Therefore, the provisional diagnoses were perforated gall bladder, traumatic liver injury, and ruptured hepatic lesion.
As the patient later on became vitally unstable, he was taken to the operating room, and the surgery started first with laparoscopic exploration. A massive intra-abdomen blood collection with active hepatic lesion bleeding taking place in segment V of the liver was found. A conversion to a laparotomy was done and the bleeding was controlled by wedge resection of segment V that included a 3.5 cm active bleeding mass. The patient's nodular liver surface was noted during the procedure (Figures 2, 3).
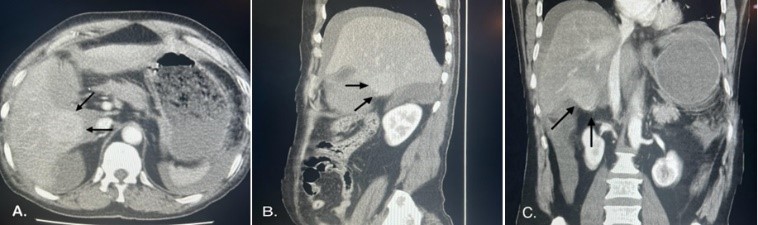
Figure 1: Computed tomography of the abdomen showing a well-circumscribed mass bulging exophytically from the inferior aspect of segment V: A) axial cut. B) Coronal cut. C) Saggital Cut.
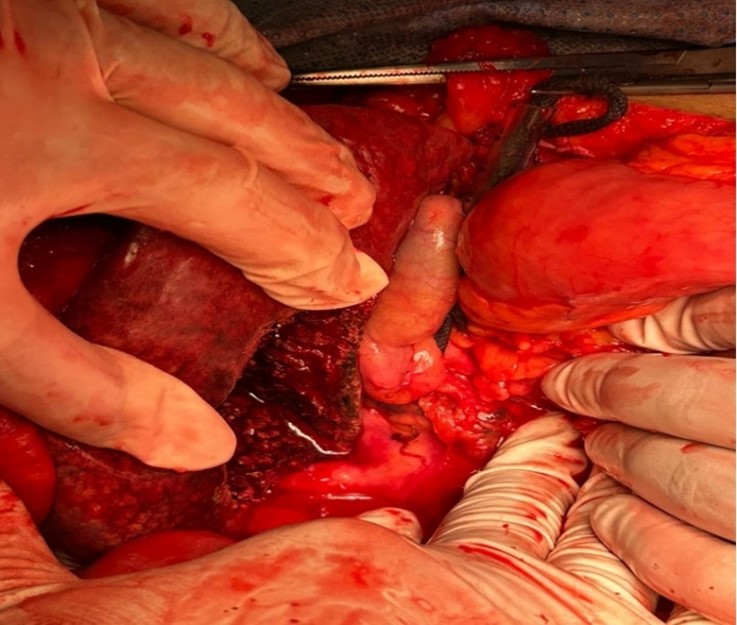
Figure 2: Operation image of the perforated lesion of segment V of the liver.
Regarding his post-operative course, the patient was admitted to intensive care unit for 3 days. He was kept (Nothing by Mouth) NPO and on intravenous fluids (IV) on the form of D5 ½ normal saline at a rate of 120cc/hr. He was given IV Paracetamol (1 g Q6h), Omeprazole (40 mg OD), Metronidazole (500 mg TID), Tazocin (4.5g Q6h), and Tramadol (50 mg Q8h). A complete blood count, liver profile and coagulation profile were taken afterwards and were within the normal range. Praziquantel 40 mg/kg (3000 mg) was given post operatively in one single dose for schistosomiasis treatment. The patient had two drains placed: a subhepatic and pelvic drain. Subhepatic drain on the first day produced 100ml heamoserous fluid, followed by 300ml of seroanguanous fluid on the 2nd and 3rd day. From day 4 to day 7 the subhepatic drain produced bile content of 15 ml, 5 ml, 10 ml and 10 ml, respectively. On day 8 post operatively, the subhepatic drain was removed while the pelvic drain continues to produce 50ml heamoserous fluid and was taken out after 2 days.
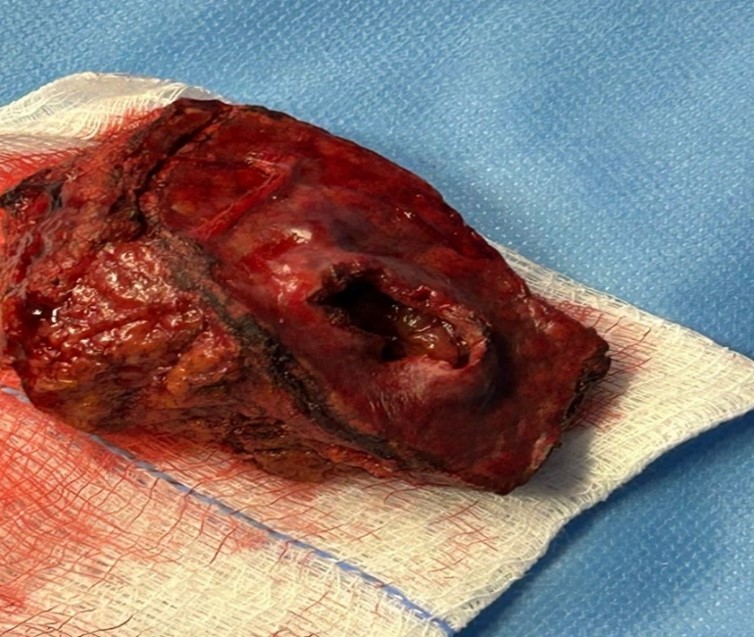
Figure 3: Resected segment V of the liver.
Regarding his histopathological analysis of the surgical resection specimens, it showed a well-differentiated HCC arranged in trabecular and pseudoscalar growth patterns and negative parenchymal margin with a diameter of 3.5 cm. Also, a vascular portal triad and visceral peritoneum invasion were identified (Figures 4 and 5). The patient stayed for a total of 17 days and was discharged with follow ups at King Abdullah Medical City in Mecca.
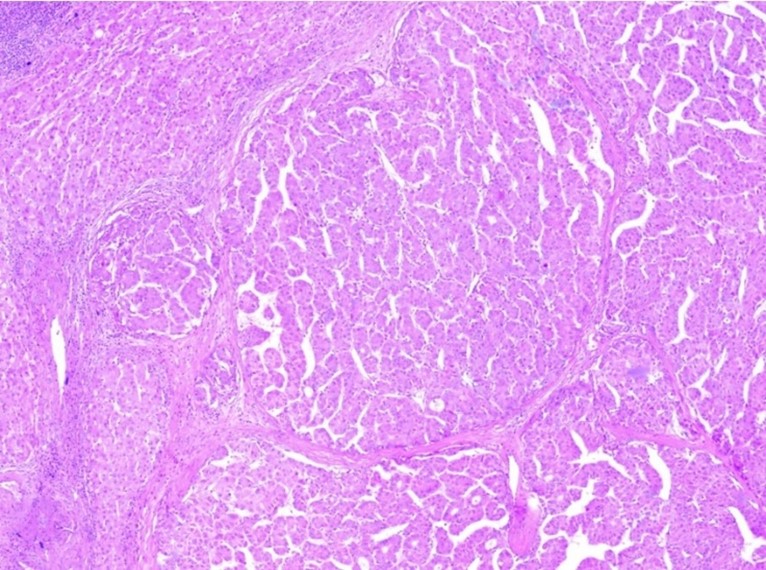
Figure 4: Hepatocellular carcinoma showing multinodular growth pattern. The nodules comprise neoplastic hepatocytes arranged in trabeculae and pseudoglandular structures (hematoxylin-eosin stain, x40).
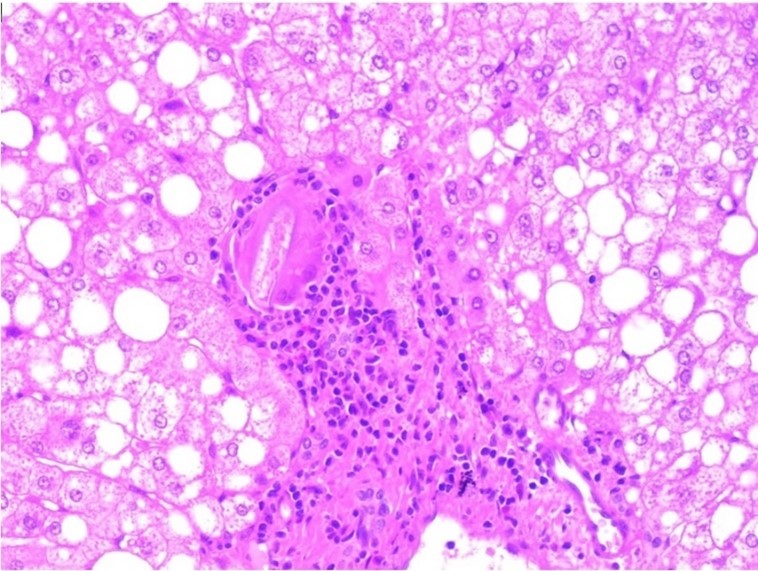
Figure 5: Schistosomial egg (S. Mansoni) surrounded by foreign body-type multinucleated giant cells and chronic inflammatory cells is identified in a portal tract adjacent to the HCC (hematoxylin-eosin stain, x200)???????
Discussion
The presented case describes a rare and challenging scenario of a ruptured HCC (rHCC) complicated by a massive abdominal hemorrhage. The incidence of rHCC is higher in Asia and African regions (3-26%) compared to Western countries (less than 3%) (6). Around 66-100% presents with abdominal symptoms while shock occurs in 33% of the cases as well as abdominal distension leading to a high mortality rate (8). Furthermore, it is estimated that more than 200 million people are affected by Schistosomiasis with most of the infected being asymptomatic (11). The interaction of schistosome egg antigens and hepatic stellate cell activation is complex. Several host regulators of fibrosis, both inhibitory and enhancing, have been identified (12). This partially explains why a few infected individuals progress towards hepatic fibrosis. A better understanding of these interactions could lead to improved resolution of fibrotic liver disease, including that associated with schistosomiasis (13).
Infections can initiate or promote carcinogenesis by three main mechanisms. First mechanism is a chronic inflammation due to the persistent infections in the host which encourages the release of oxidative and nitrogenous reactions resulting in the damage of DNA, protein, cell membranes and changes enzyme activity and gene expression, possibly leading to carcinogenesis. The second mechanism is the insertion of oncogenes into the host genome (Hepatitis B or C), inhibition of tumor suppressors, or stimulation of mitosis. Lastly, inducing immunosuppressors and reducing immunosurveillance. Regarding Parasites such as schistosomiasis infections, it usually initiates neoplastic changes via the first mechanism (14).
The etiological factors responsible for rupture are not fully understood; however, several hypotheses have been presented in the literature. The small room hypothesis demonstrated by Li et al., in their case series of 89 ruptured HCC patients, revealed that when tumor growth exceeds its capacity, it leads to rupture of parenchyma and tear in the capsule (15). Previous studies have shown that an HCC size of more than 5 cm elevates the risk of rupture (16, 17). However, in the present case, the HCC size was 3.5 cm. Therefore, size alone does not explain HCC rupture fairly. Tanaka et al., in their study, reported an HCC size of 2 cm that was also ruptured (18). Another hypothesis proposed by Lixin et al. implicates vascular injury of arterial walls supplying to the tumor in HCC rupture (19). The venous congestion hypothesis considers occlusion of hepatic veins as the main cause of increased pressure within the tumor, leading to subsequent rupture (20). Trans-arterial Chemo-embolization (TACE) has also been considered to increase the risk of HCC rupture (20, 21). Vascular injuries related to TACE can explain the higher likelihood of HCC rupture in such patients (22). Zhu et al., in their study, also identified hypertension, extrahepatic invasion, and liver cirrhosis as independent factors for HCC rupture (16). However, the present case had no history of prior medical illness.
In the present case, clinical examination and laboratory investigations revealed signs of significant intra-abdominal bleeding and liver dysfunction. Our patient was initially presented with right upper quadrant pain. Acute abdominal pain is a key clinical feature that has been reported in approximately 66-100% of HCC rupture patients (6). Furthermore, a physical examination of our patient also revealed distention with guarding and significant tenderness. About one-third of HCC rupture patients present with abdominal distention (22). On CT examination, slightly hyperdense fluid was noted around the liver, right side abdomen, and in the pelvis, suggestive of most likely hemorrhagic fluid. The utility of CT examination is an integral part of the diagnosis process in HCC rupture cases, with around 66% of cases showing hyperechoic areas around the tumor (23). Hemorrhage secondary to HCC rupture is quite difficult to diagnose and manage. If it is not detected and managed early, it can lead to fatal outcomes. As the prognosis of such cases is often poor, it is also essential to distinguish these cases from other types of liver dysfunction (24). The surgical approach in this case involved a combination of laparoscopy and laparotomy. In their cases of abdominal hematoma and shock, Mozafari et al. identified the region of the left lobe of the liver as a source of bleeding (25).
rHCC is associated with a high in-hospital mortality rate of 25% to 75% (26). It was reported by Tanaka et al. that rHCC should be differentiated when it occurs in the early phase of cirrhosis rather than in the late phase (18). The prognosis is strongly determined by the functional status of the liver immediately after bleeding. A study done by Liu et al. (15) reported that the survival of patients with rHCC was significantly worse than those with Non rHCC (7). However, Yeh et al. reported that patients with non-ruptured HCC had a similar overall survival as those with rHCC but the disease-free survival rate was significantly better (27). Mizuno et al. tried to compare the survival rates of patients with ruptured and non-ruptured HCC based on the same background factors, such as disease stage and liver function (26). They found no significant difference in the 2 groups of patients in the overall survival and disease-free survival (26). Interestingly in another study, patients who underwent anti-tumor therapy before HCC rupture had poor survival (HR = 4.36, 95%CI: 1.27-14.99, P = 0.019). These therapies included transhepatic arterial chemoembolization (TACE) (24), surgical treatments (28), radiofrequency ablations (28), TACE combined with oxaliplatin chemotherapy (28), and TACE combined with sorafenib (29). The overall survival rate of patients with rHCC who did not undergo anti-tumor therapies before tumor rupture was significantly increased compared with those who did (30). Early treatment of schistosomiasis disease produces better outcomes, even those affected by a hepatic, urinary, or neuro-cerebral disease. Hepatosplenic Schistosomiasis has a relatively good prognosis with preserved hepatic function until the end of the disease, without variceal bleeding. Late-stage treatment provides less benefit with multiple system involvement, especially with a past parasitic load in tissues (31).
Similarly, Chedid et al. reported three cases of hemorrhages following HCC rupture (32). Similar to our case, all their patients also presented with abdominal pain, requiring emergency laparotomy. In one of their cases, the patient's liver was cirrhotic and enlarged, and in their second case, the patient presented with complications on follow-up, including hypovolemia, tense abdomen, and chronic liver disease. However, in most cases, as seen in the present case, the postoperative period is free from complications (25). Diagnosing ruptured HCC is quite challenging due to variability in clinical presentation. However, shock can be considered an indicator of rupture. Although there are several treatment options available in such cases, emergency laparotomy is usually deemed a go-to approach with significant positive outcomes.
Furthermore, the biopsy revealed atypical hepatocytes, dilated blood vessels, and a schistosomal egg surrounded by foreign body-type multinucleated giant cells and chronic inflammatory cells identified in a portal tract adjacent to the HCC. The hepatic schistosomiasis eggs lodge in the presinusoidal radicles of the portal vein, where they elicit a granulomatous fibrosing reaction that blocks venous blood flow. Portal hypertension results in compensatory portosystemic blood flow and late progressive liver damage (Symmers pipestem fibrosis is pathognomonic). It is distinct from cirrhosis because there is no hepatocyte dysfunction, portal hypertension results from fibrosis within blood vessels. In our case, the patient showed no signs or symptoms of portal hypertension. The biopsy results of our patient reveal a dual infection of HCV and schistosomiasis. Although the literature review is inconclusive on whether schistosomal infection alone has carcinogenic potential, it is highly likely to act as a cofactor for the hepatic lesion and potentiate injury (14).
Conclusion
This case highlights the complexity of managing a rare presentation of ruptured HCC with massive abdominal bleeding. The patient in the present case did not have any medical illness that could indicate the risk factors for ruptured HCC or hemorrhage. Prompt diagnosis and intervention were crucial in this emergency scenario. The patient underwent emergency laparoscopic exploration, which showed massive intra-abdomen blood and an active hepatic lesion was found at segment 5 of the liver, so the surgery converted to exploratory laparotomy, and bleeding was controlled with wedge resection of segment five that included 3.5 cm active bleeding mass. As some previous studies indicated, sizes above 5 cm are prone to rupture, but this case highlights that HCC rupture is not dependent on size only. Furthermore, the results of the biopsy revealed an HCV and schistosomiasis infection. In our case, no postoperative complication was noted. This case underscores the importance of early diagnosis and appropriate surgical management.
Disclosure
Conflict of interest
There is no conflict of interest
Funding
No funding
Ethical consideration
A written informed consent was taken from the patient to include the data in the form of case publication with protection of the patient’s anonymity.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, clinical data drafting, diagnosis, treatment and final writing of the manuscript.