Volume 4, Issue 2
February 2024
Assessing the Efficacy of Tranexamic Acid in Minimizing Perioperative Blood Loss in Ovarian Cancer Surgery: A Systematic Review of Randomized Controlled Trials and Meta-Analysis
Azhar M. Al Ibrahem, Basmah A. Qasem, Raneem A. Almadabighi, Dalia H. Almasud, Lujain H. Alomar, Mohammad H. Sindi, Abdulmalik S. Barakat, Reem A. Bajaaj, Alreem M. Alhaji, Sally A. Alhumaid, Rand M. Alfawaz, Nihad A. Al Kishi
DOI: http://dx.doi.org/10.52533/JOHS.2024.40204
Keywords: Ovarian cancer, ovarian neoplasms, tranexamic acid
Introduction: Ovarian cancer stands as the predominant gynecological malignancy on a global scale a global gynecological malignancy with a >60% mortality rate, often necessitates aggressive surgeries like maximal cytoreduction to enhance survival. However, these procedures pose bleeding risks requiring blood transfusions. Tranexamic acid (TXA), a long-established clotting inhibitor, offers potential solutions. Our objective is to assess TXA's safety and efficacy in minimizing perioperative bleeding during ovarian cancer surgery through a thorough literature review.
Methods: This systematic review adhered to PRISMA guidelines. It encompassed RCTs involving women aged 18+ undergoing ovarian cancer surgery with tranexamic acid versus placebo or no treatment. We conducted a comprehensive electronic search in PubMed, Cochrane Library, and MEDLINE using MeSH terms and related keywords to identify relevant studies, with no language limitation.
Results: We focused on four RCTs spanning from 2008 to 2023, exclusively involving females, with group sizes ranging from 60 to 150. The tranexamgroupsid group exhibited a notable reduction in perioperative bleeding and required fewer blood transfusions than the control group. However, no remarkable variances were identified in vomiting, hematoma, or bleeding rates between the two groups. These findings offer valuable insights with implications for medical practice and future research.
Conclusion: In conclusion, A systematic analysis of randomized controlled trials related to surgical treatments in ovarian cancer reveals robust evidence favoring tranexamic acid for reducing both intraoperative and the need for blood transfusion. However, future research should include long-term follow-up studies to gauge its influence on patient survival, disease recurrence, and other vital clinical parameters.
Introduction
Among all the cancers related to a woman's reproductive system, ovarian cancer is the third most popular one (1). Sixty percent probability of death if an individual is exposed to it. If Ovarian cancer reaches to advanced stage, then surgery is required to remove the damaged organs (2). The established treatment approach for advanced epithelial ovarian cancer (EOC) involves cytoreductive surgery and platinum-based chemotherapy. Cytoreductive surgery, commonly known as debulking, seeks to eliminate not just the ovaries but also the uterus, fallopian tubes, and as much visible tumor mass as feasible. Research indicates that the extent of remaining disease post-cytoreductive surgery significantly influences the independent prognostic factor for survival among women with EOC (3). Although there will be improvements, there will be serious risks such as high blood loss, so allogeneic blood transfusion is required, but this process – allogeneic blood transfusion – has some undesirable outcomes such as impaired renal function, possible infection, immunological variance and in worst cases is life-threatening outcomes such as death (4). Cytoreductive surgery is a comprehensive procedure, and around 4.1% to 7.4% of individuals with advanced EOC encounter significant bleeding during the operation, leading to a transfusion rate of 30% to 40% for red blood cells (5).
A medication preventing the breakdown of blood clotting by stopping the work of Plasma is called Tranexamic acid (TXA). This has been utilized for more than 40 years. Anyhow, venous thromboembolic events (VTE) – that take place when blood clots close a vein - can appear in the case of more advanced stages of Ovarian cancer. (VTE) is caused when there is no adequate control (TXA) that has a high risk on a patient which threatens his/her life. As a result, controlling the reduction of bleeding & complications is significant in the case of using (TXA) during the procedure (6–9). In emergency and urgent surgical situations, the use of tranexamic acid resulted in a 30% reduction in the likelihood of requiring a blood transfusion (10). In trauma cases, the administration of tranexamic acid resulted in a 15% reduction in the risk of death from bleeding (11). Postpartum blood loss was found to be reduced in women who received tranexamic acid compared to those who received a placebo, with a mean difference of 75.17 mL; 95% CI 108.23 to 42.12 mL). This reduction was observed irrespective of the mode of delivery (12). Furthermore, when contrasted with a placebo, tranexamic acid proves to be a successful intervention in diminishing blood loss during myomectomy procedures for fibroids (8).
Currently, limited literature has evaluated the safety and efficacy of tranexamic acid in treating late-stage ovarian cancer in women. Even though the literature presents limited evidence in treating advanced ovarian cancer in women, the safety and effectiveness of tranexamic acid remain undefined and incomplete (13,14). The effectiveness of taking one single dosage of preoperative intravenous TXA is presented in only one research in which (15 mg/kg) dosage is given in cytoreductive surgery. Nonetheless, the reduction of bleeding is still not known when considering the efficacy and safety of multiple TXA (Tranexamic Acid) regimens (15,16).
Our objective is to address these research gaps by conducting a systematic review of the latest studies to assess the safety and efficacy of tranexamic acid in minimizing perioperative blood loss during late-stage ovarian cancer surgery.
Methods
Systematic review
Literature Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria were followed for conducting this systematic review (17). The International Prospective Register of Systematic Reviews (PROSPERO) has the study protocol listed under (ID: CRD42023453079) (18). A thorough electronic search was carried out in the databases PubMed, Cochrane Library, MEDLINE, and Embase in August 2023. To find pertinent papers, Medical Subject Heading (MeSH) terms and associated keywords were combined. The search terms included variations of "ovarian cancer" (e.g., "ovarian neoplasms," "ovary neoplasms") and "tranexamic acid" (including synonyms such as "tx"). The search encompassed studies without any limitations on publication dates.
Inclusion/Exclusion Criteria
This review included Randomized Controlled Trials (RCTs) containing information on women aged 18 years or older who underwent ovarian cancer surgery and were administered tranexamic acid during the surgery, in comparison to placebo or no treatment. There were no restrictions based on language. Exclusion criteria included any of the following studies: that were not randomized controlled trials, did not report outcomes. of interest for the clinical questions, included pediatric patients or patients under 18 years old, did not involve using tranexamic acid during ovarian cancer surgery, Studies with a high risk of bias or low quality.
Study Selection
All records resulting from the primary search were imported to Mendeley to facilitate the removal of duplicate entries. The deduplicated results were subsequentially imported to Rayyan, where they underwent an initial screening for relevance based on the information presented in the titles and abstracts. The screening process was carried out by 5 authors to determine which studies met the predefined inclusion and exclusion criteria.
Following the initial screening, the full texts of the studies that passed the first stage were retrieved and thoroughly examined by 5 authors to make the final determination regarding the inclusion or exclusion of each study.
Data extraction and analysis
Study identifications, such as author(s), year of publication, country of origin, study design, sample size, follow-up duration, and inclusion/exclusion criteria, were included in the data retrieved from the preserved studies. Additionally, participant characteristics were recorded, such as age and sex distribution, relevant medical history, medications used, preoperative hemoglobin level, and presence of bleeding disorders. Intervention characteristics encompassed the dosage of tranexamic acid, timing of administration, and mode of delivery. The outcome measures category involved the documentation of intraoperative blood loss, postoperative bleeding occurrences, transfusion rate, incidence of thromboembolic events, adverse effects related to the intervention, and length of hospital stay. Lastly, the study results section covered the key findings reported in the study, limitations acknowledged by the authors, and any recommendations or implications for future research.
Quality of the included studies and the risk of bias assessment: For randomized controlled trials, we made use of Cochrane's risk of bias tool. Studies that pose a severe randomized bias risk will not be included (19). This will entail assessing each study considering several criteria, categorizing them as having a low, high, or uncertain risk of bias for each.
Statistical analysis
All analyses were conducted using RevMan (version 5.4.1; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) (20). We extracted the means and standard deviations of the scores for the questions evaluating improvements in quality of life from the included studies, both pre-and post-liposuction. A weighted mean difference with 95% confidence intervals (CIs) was pooled using a fixed-effects model. Forest plots were created to evaluate the results of pooling. P value less than 0.05 was considered significant; Heterogeneity between trials was assessed using the Higgin I2 test according to the Cochrane Handbook.
Results
Search result
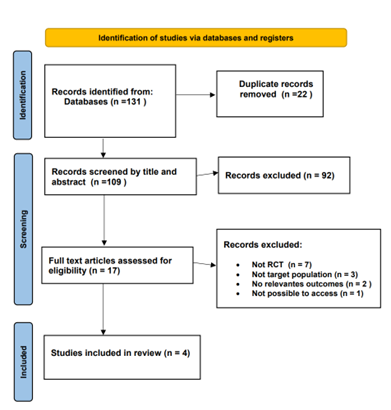
A comprehensive review of 131 relevant articles was conducted, comprising 86 from PubMed, 41 from Cochrane, and 6 from Medline. After applying specific inclusion criteria, the focus was narrowed down to 17 articles for in-depth analysis. From this selection, four randomized controlled trials (RCTs) conducted between 2008 and 2023 were included. The PRISMA flow chart depicts the process of the literature screening, see (Figure 1).
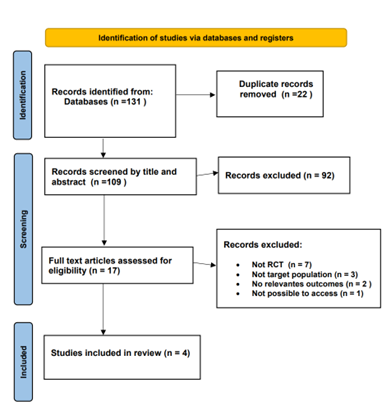
Figure 1: Flow Diagram of preferred Reporting items For Systematic Review and Meta-Analysis.
Risk of bias assessment
The Cochrane Collaboration for Systematic Review of Interventions was approached to evaluate the RCTs' quality. All the randomized controlled trials (RCTs) Provided specific criteria for inclusion and exclusion and outlined a randomization methodology: two of the studies were double-blind randomized clinical trials; one was a prospective-randomized clinical trial; one was a randomized controlled clinical trial; and one was randomized comparative. Our assessment of these RCTs, following Cochrane Collaboration standards, is visually presented in (Figure 2) and (Figure 3).
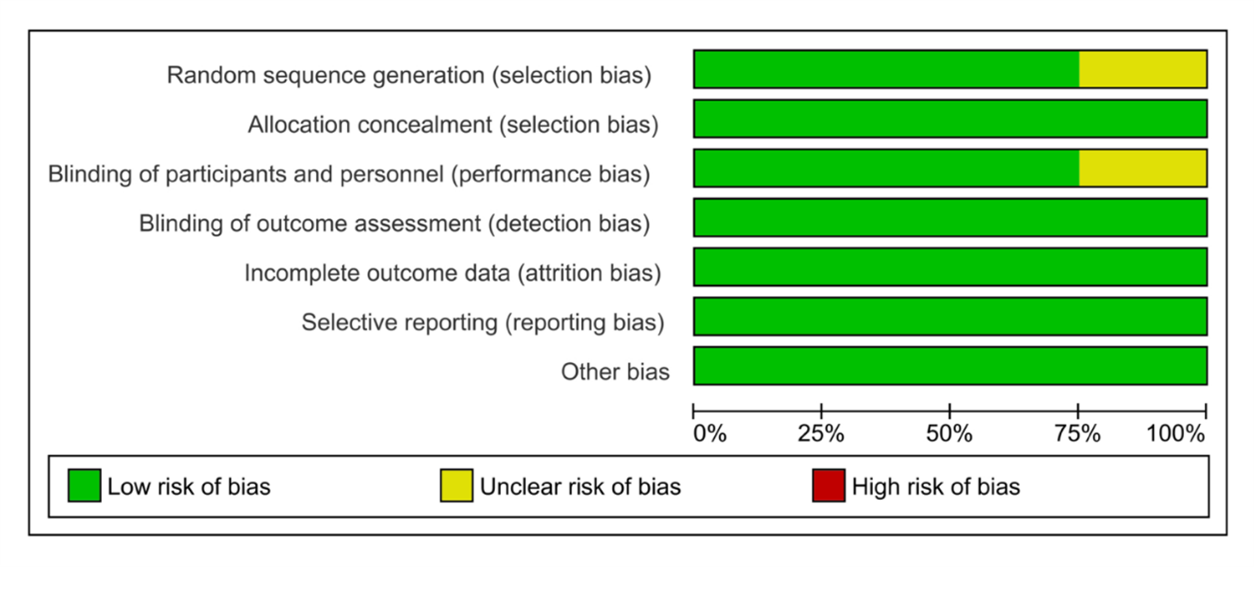
Figure 2: Results of the risk of bias analysis
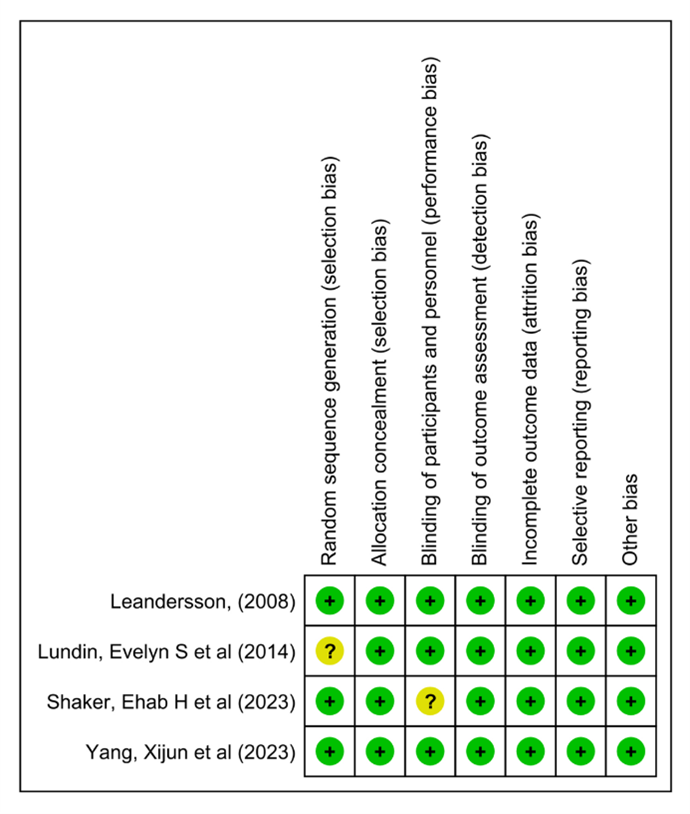
Figure 3: Summary of the risk of bias.
Study characteristics
The sample size of the included studies fluctuates between 60 and 150 individuals. All of them underwent Ovarian Cancer Surgery, the intervention groups were administered intravenously TXA with a dosage ranging from 10mg/kg to 15mg/kg, while the control groups received Sodium Chloride (NaCl), The anesthesia administration protocol was not standardized. The follow-up period varied from 4 weeks to 3 weeks, only one article did not report follow-up duration, reported that the count of peri-operative blood loss (ml) procedure was performed for both group, and hospital stay not reported by all the 4 studies, The results of intervention group characteristics are summarized in (Table 1).
|
Table (1). Characteristics of Studies included in the Review. |
||||||
|
Study |
Country |
Reference type |
No. of participants |
Age (yr.) |
TXA dosage |
Focus |
|
Yang, 2023 |
China |
RCT |
150 |
H dose 54.9 ± 8.1 L dose 53.2 ± 8.3 |
H dose 20mg/kg L dose 10mg/kg |
Use of high-dose tranexamic acid in surgery. |
|
Shaker, 2023 |
Egypt |
RCT |
60 |
11.02 ± 44.42 |
10mg/kg |
Use of tranexamic acid in CRS followed by HIPEC. |
|
Lundin, 2014 |
Sweden |
RCT |
100 |
82-33 |
15mg/kg |
Use of tranexamic acid in advanced ovarian cancer. |
|
Leandersson, 2008 |
Sweden |
RCT |
100 |
63 |
15mg/kg |
Use of Single dose tranexamic acid in ovarian FIGO stage II-IV in cytoreductive radical surgery. |
The results are noteworthy, indicating a significant reduction in perioperative blood loss (measured in milliliters) in the group receiving tranexamic acid when compared with the control group. Additionally, the tranexamic acid-treated group required fewer blood transfusions, underscoring the efficacy of tranexamic acid. However, no significant differences were identified in terms of vomiting, hematoma, or bleeding between the treatment and control groups.
Results of the Meta-Analysis
Among the four studies meeting our selection criteria, we initially focused on the effect of tranexamic acid on the need for blood transfusion, forest plot shown in (Figure 4). The TXA intervention group had 224, while the control group was made up of 225 participants. Statistical analysis revealed (Chi2= 4.46, df=3) with an I 2 static of 33%, minimal heterogeneity. The overall effect was statically significant with Z= 2.65 (P=0.008), supporting the favorable effect of TXA in reducing the need for blood transfusion in patients who underwent ovarian cancer surgery.
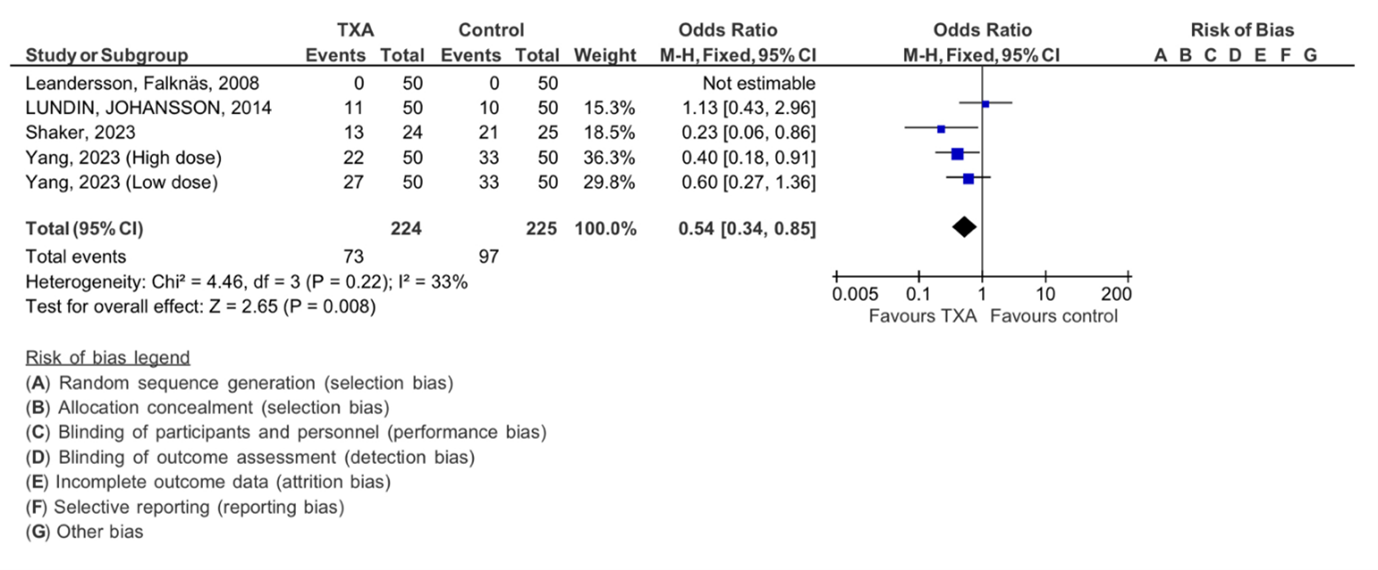
Figure 4: Meta-analysis of outcomes concerning the influence of tranexamic acid on the need of blood transfusion. The analysis utilized a Mantel-Haenszel random-effects model, presenting odds ratios along with 95 percent confidence intervals. TXA refers to tranexamic acid.
We examined the intraoperative blood loss, forest plot shown in Figure 5. In this analysis, four studies were included, with a total of 224 subjects in the TXA intervention group and 225 subjects in the control group. The analysis revealed no significant heterogeneity (Chi2= 11.68, df=4, P=0.0.02), with an I 2 statics of 66%, indicating moderate heterogeneity. The overall effect was found to be statistically significant with Z=4.21 (P < 0.0001), supporting the effect of TXA in minimizing intraoperative bleeding in our participants.
Additionally, the thromboembolic events in our Four included studies are shown in (Figure 6). The analysis demonstrated no significant heterogeneity (Chi2= 1.90, df=1, P=0.17), with an I 2 statics of 0%, indicating no heterogeneity. The overall effect was found to be insignificant with Z=1.61 (P = 0.11).
In summary, the results are noteworthy, indicating a significant reduction in perioperative blood loss (measured in milliliters) in the group receiving tranexamic acid, when compared with the control group. Additionally, the tranexamic acid-treated group required fewer blood transfusions, underscoring the efficacy of tranexamic acid. However, no significant differences were identified in terms of thromboembolic events when compared to the control group.
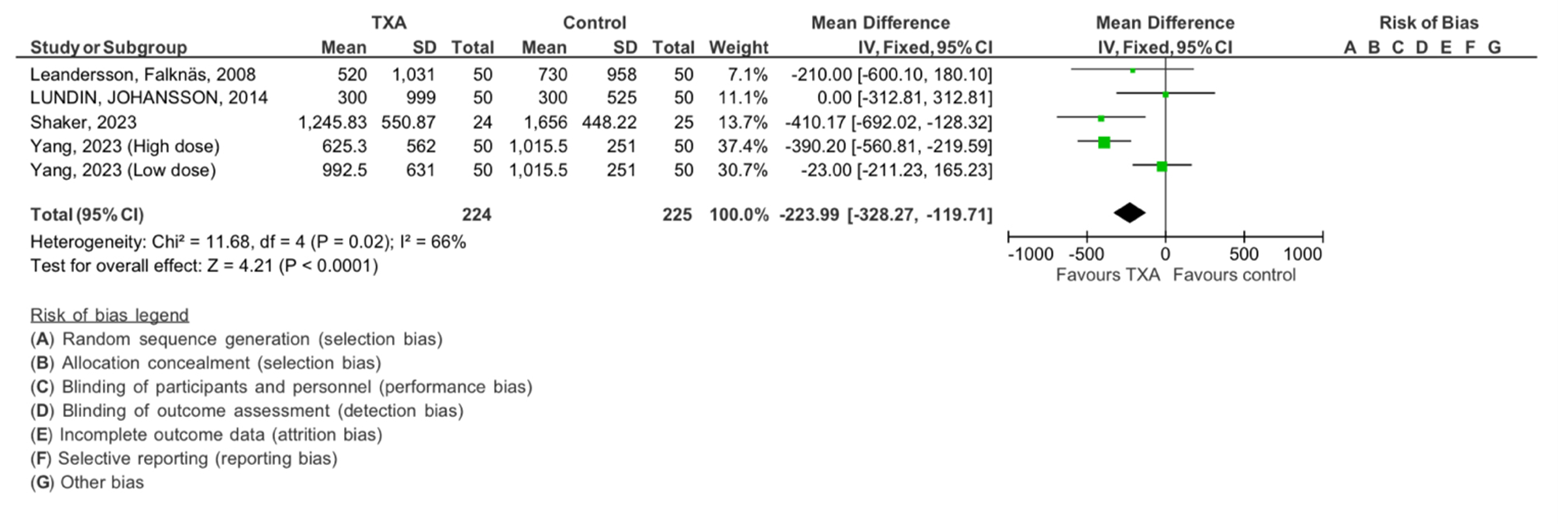
Figure 5: The meta-analysis results concerning the impact of tranexamic acid on intraoperative blood loss. The analysis employed an inverse-variance random effect model. Mean differences, accompanied by 95 percent confidence intervals, are presented. The values represent mean values with standard deviations. TXA refers to tranexamic acid.
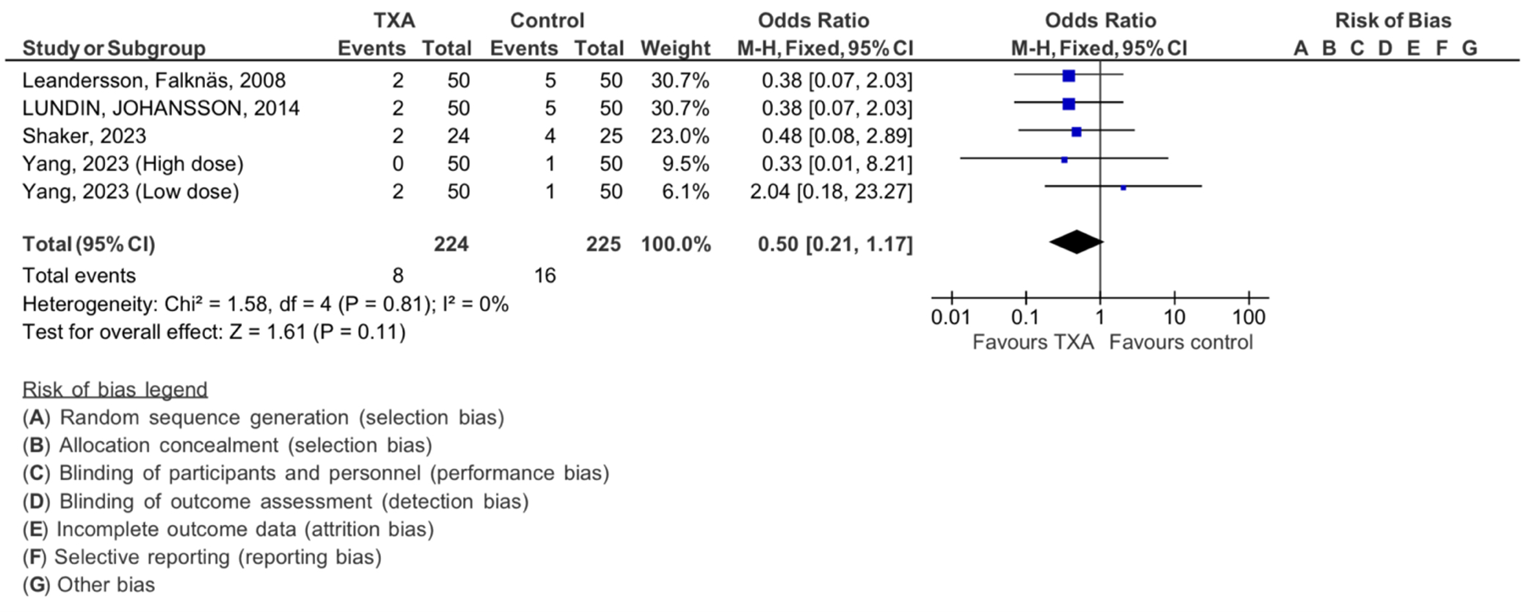
Figure 6: Meta-analysis of outcomes concerning the influence of tranexamic acid on thromboembolic events. The analysis utilized a Mantel-Haenszel random-effects model, presenting odds ratios along with 95 percent confidence intervals. TXA refers to tranexamic acid.
Discussion
Ovarian cancer is a malignancy affecting the ovaries, critical reproductive organs in females. Surgical interventions for ovarian cancer entail inherent risks of blood loss, occasionally necessitating blood transfusions.
A recent systematic review and meta-analysis aimed to evaluate the potential efficacy of tranexamic acid in reducing blood loss during ovarian cancer surgery. Tranexamic acid is a pharmacological agent that minimizes excessive bleeding by slowing the breakdown of blood clots. The evaluative process involved a comprehensive examination of existing research studies on this subject.
The research findings strongly indicate that administering tranexamic acid to female patients during ovarian cancer surgery is significantly more effective in reducing blood loss when compared to a placebo or no intervention. This is of great clinical importance, given the inherent risks and complications linked to blood transfusions. Consequently, the systematic review and meta-analysis provide compelling evidence supporting the use of tranexamic acid as a sensible approach for managing blood loss during ovarian cancer surgery.
The beneficial effect of tranexamic acid for minimizing perioperative blood loss during ovarian cancer surgery is evaluated in this systematic review and comprehensive meta-analysis by conducting a literature review. Studies demonstrated that tranexamic acid, when administered during surgery on female patients, is more effective at preventing blood loss and the need for a blood transfusion than a placebo or no treatment.
Because ovarian cancer surgery frequently presents bleeding difficulties, administering tranexamic acid decreases the amount of blood lost after the operation and eliminates the need for a blood transfusion. We observe that the effectiveness of tranexamic acid fluctuates based on whether the ovarian cancer is unilateral or bilateral, the dose, duration, and quality of the surgery, and the duration of the review because the benefits of the drug are more evident when the review period exceeds one month.
According to the randomized controlled trials (RCTs) assessed, between 40 and 150 female ovarian cancer patients were randomly selected from a group of ovarian cancer patients. The 2021 study compared the levels of blood loss and AMH before and after surgery, and the comparison presented unilateral or bilateral ovarian cancers. The level of AMH decreased for patients who took the drug, and it was suggested that the results might be more effective if the drug's dosage was increased, and the review period extended to 3 months (13). In contrast, in the 2023 study, the review lasted for one month, and the results were reviewed within days, resulting in a decrease in the rate of blood loss. Less blood transfusion is needed; nevertheless, some patients persist with difficulties. Acute respiratory distress syndrome was brought on by increased coagulation. Because cancer patients' impaired immune systems make blood transfusions risky, the results were satisfactory (21). They were split into three groups and compared to a group taking medicine in a different trial. In one placebo group, one received a low dose of the medication, while the other received a high dose. According to the study, patients who obtained the higher dose proved to be more effective (4). Another study from 2013 evaluated whether ovarian cancer patients should receive an intravenous dose before the operation to prevent blood loss and minimize blood transfusions. It was found to be beneficial as the first line of prevention of bleeding in cases of advanced ovarian cancer (15).
Evaluating it indicates how well the drug acts depending on the type and size of the tumor. A high intravenous dose administered just before surgery is the most efficient strategy to minimize blood loss and the need for blood transfusions, which may cause coagulation problems depending on patient characteristics and medical history.
Another study published in 2008 calculated the extravasation of hemoglobin, assuming allogenic transfused hemoglobin. The patient's pretreatment hemoglobin levels and overall blood loss were connected. Also taken into consideration was blood loss due to hematomas or reoperation within the first five days following surgery. In women having surgery for advanced ovarian cancer, a single dosage of tranexamic acid administered intravenously beforehand reduces the amount of perioperative bleeding and lessens the requirement for blood transfusion (22).
Assessing the drug's efficacy according to tumor type and size, administering a high intravenous dose right before surgery is the most effective method to reduce blood loss and the need for blood transfusions. However, potential coagulation issues must be considered, depending on individual patient characteristics and medical history.
One of the primary limitations is the relatively small number of RCTs that met the inclusion criteria, and variability in Patient Characteristics: The patient populations in the studies included in the analysis might vary in terms of age, underlying health conditions, tumor features, and other factors. These differences could affect how applicable the findings are to diverse groups of patients.
Moreover, there is an absence of long-term data on outcomes. The review mainly concentrated on immediate surgical outcomes like blood loss, transfusion rates, and immediate post-surgery complications. Endpoints with longer-term implications, such as disease recurrence, overall survival, and quality of life, were not examined. Consequently, the effects of TXA on these crucial clinical aspects remain uncertain.
Conclusion
Our systematic review of randomized controlled trials regarding tranexamic acid use in late-stage ovarian cancer surgeries provides strong evidence supporting its efficacy in decreasing perioperative hemorrhage. Across multiple studies, it consistently demonstrates a significant reduction in both intraoperative and postoperative bleeding among ovarian cancer patients when compared with patients who received a placebo or no intervention. For optimal results, the administration of tranexamic acid should involve high dosages immediately before surgery. The reviewed studies also reveal that various factors, including tumor size, tumor type, unilateral or bilateral cyst presence, duration of surgery, and pre-treatment hemoglobin levels in patients, influence the effectiveness of tranexamic acid. Depending on individual patient differences, potential complications such as coagulation problems and acute respiratory distress should be considered.
Furthermore, there is a requirement for studies with prolonged follow-up periods to evaluate the effects of tranexamic acid on patient survival, disease recurrence, and other critical clinical outcomes. While tranexamic acid appears to be a valuable tool in ovarian cancer surgery, further research is essential to fine-tune its role in managing this complex condition.
Disclosure
Statement
The authors declare no conflict of interest.
Funding
None.
Financial Support
None.
Ethical Consideration
The need for ethical approval was waived due to the nature of the study.
Data availability
Data is available upon request from the corresponding author.
Author Contribution
Leader: Dr. Azhar M. Downloaded previous articles and checked for duplicates, led the development of the systematic review protocol, assisted with the design data extraction sheet, and submitted the manuscripts. Basmah A. and Raneem A. assisted with assessing the risk of bias and writing the discussion section. Lujain H. and Sally A. assisted in Writing the introduction. Dalia A. wrote the methods section, and She took the lead role in Rearrange references. Mohammad H. Alreem M. conducted the meta-analysis and wrote the results section. Abdulmalik B. took a lead role in Writing the conclusion. Reem A. and Mohammad H. assisted with the formulation of the abstract. Rand M. writing the cover letter. Dr. Nihad Al Kishi supervised the work. All authors Reviewed full texts of eligible studies Proofread and contributed to the final manuscript.