Volume 4, Issue 1
January 2024
Comprehensive Overview of Denture Stomatitis from Etiology to Prevention
Haitham Binhuraib, Aliya Alqhatani, Haifa Alhasher, Sarah Alshehri, Arwa Alhudiry, Fatmah Alqhtani, Deema Almohaya, Rahaf Alqahtani, Ahmed Alobaid, Aseelah Alshahrani, Rana AlHajari, Ashwaq Alreshide
DOI: http://dx.doi.org/10.52533/JOHS.2024.40112
Keywords: denture stomatitis, Candida albicans, oral hygiene, denture care, antifungal treatment, prosthodontics
Denture stomatitis (DS) is an oral mucosal inflammation prevalent among denture wearers, characterized by diffuse or localized erythematous inflammation. Its occurrence, affecting 20%–80% of denture users globally, is nearly equal across genders, with a slight female predominance. The condition's etiology is multifaceted, involving poor oral and denture hygiene, continuous denture wear (especially overnight), and ill-fitting dentures leading to trauma. Candida albicans, along with other microbial agents, play a significant role in DS pathogenesis, facilitated by an environment conducive to fungal growth. Treatment and prevention strategies focus on patient education, behavior modification, and routine dental care. These include daily mechanical cleaning and appropriate disinfection of dentures, periodic professional maintenance, and avoiding constant denture wear. The review emphasizes the importance of distinguishing cleaning from disinfection and sterilization, highlighting effective methods like immersion in sodium hypochlorite solutions and microwave irradiation. Despite the efficacy of antifungal treatments, their use is limited due to the potential for recurrence. The review underscores the necessity for continuous commitment to denture hygiene and the benefits of using high-quality prosthodontic materials for denture maintenance and replacement. A comprehensive approach to managing DS contributes to the long-term resolution of clinical signs and reduces health risks in vulnerable populations.
Introduction
Denture stomatitis (DS) is identified as an inflammation of the oral mucosa in areas where dentures rest, presenting as either diffuse or localized erythematous inflammation (1). This condition is remarkably common, affecting a significant portion of denture wearers, with its prevalence ranging between 20% and 80% globally (1-3). The condition's equal impact on males and females, with a slight inclination towards females, indicates the necessity for gender-specific considerations in treatment and prevention strategies (4). Its etiology is complex and multifactorial, involving various determinants such as inadequate oral and denture hygiene, the habit of wearing dentures continuously, especially at night, contamination of the dentures with mature polymicrobial biofilms containing Candida, and the ill-fitting of dentures, which leads to trauma in the denture-bearing area (5). DS is a significant oral health concern, primarily due to its high prevalence among denture users. Its wide-ranging global distribution underscores the need for universal awareness and effective management strategies.
The likelihood of developing DS increases among individuals who smoke, older adults, and those who use older dental prostheses (6). Treatment strategies for DS primarily focus on behavioral modifications at the patient level. Key recommendations include routine cleaning and disinfection of the dentures on a daily basis and avoiding the constant wearing of dentures, particularly 24 hours a day. While antifungal medications can be beneficial as adjunctive treatments, their effectiveness tends to be limited to short-term use. The multifactorial nature of DS necessitates a comprehensive approach to its management. Factors such as poor hygiene practices related to both the oral cavity and dentures are crucial in the development and perpetuation of this condition. Moreover, the habitual wearing of dentures, especially at night, has been identified as a significant contributing factor. This practice not only promotes the growth of pathogenic biofilms but also increases the risk of mucosal trauma due to prolonged pressure and friction against the oral tissues. This aspect of behavior modification is central to the treatment and prevention of DS. Encouraging patients to remove their dentures during sleep and ensuring proper cleaning and maintenance can significantly reduce the incidence and severity of the condition (6). The use of antifungal medications, although beneficial in the short term, highlights the need for a more sustainable and preventive approach to management. The limited long-term efficacy of these medications necessitates a greater focus on preventive measures and lifestyle modifications. This review article provides a comprehensive overview of the various aspects of DS, offering insights into epidemiology, etiology, treatment, and prevention, thus serving as a valuable resource for healthcare professionals in the field of oral health.
Review
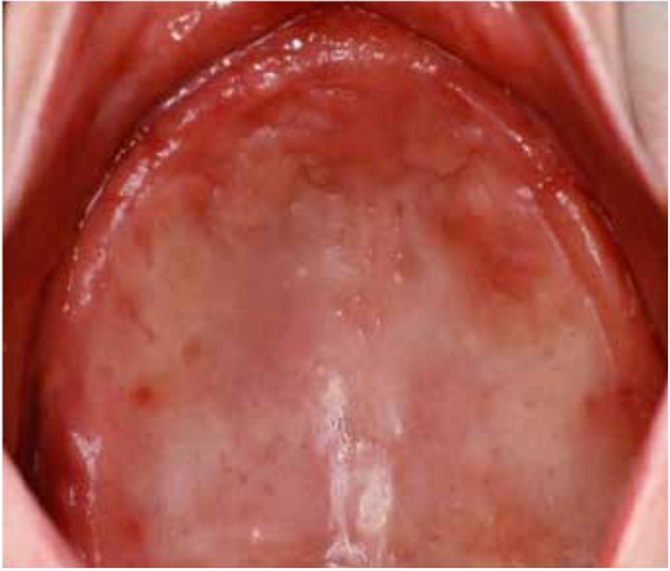
DS primarily impacts the upper arch, especially under maxillary complete dentures, due to its unique microenvironment and reduced exposure to saliva and tongue movements (1, 7, 8). Rarely affecting the lower arch, DS is classified based on the appearance of the inflamed mucosa beneath the denture. Newton's classification includes Type I (localized inflammation) (Figure 1), Type II (generalized erythema) (Figure 2), the most common form, and Type III (inflammatory papillary hyperplasia) (Figure 3) (9). This classification was later refined by Budtz-Jorgensen and Bertram, focusing on the type of inflammation present (10). These classifications aid in understanding and treating the varying presentations of DS.
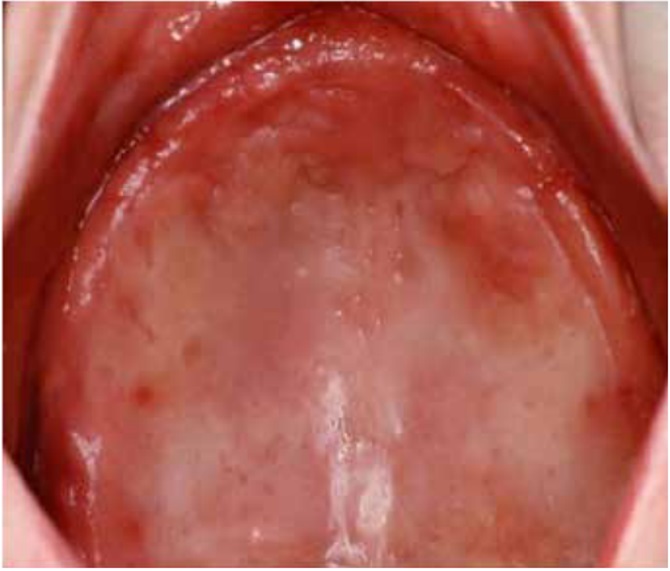
Figure 1: Newton’s Type I DS showing areas of localized inflammation (13)
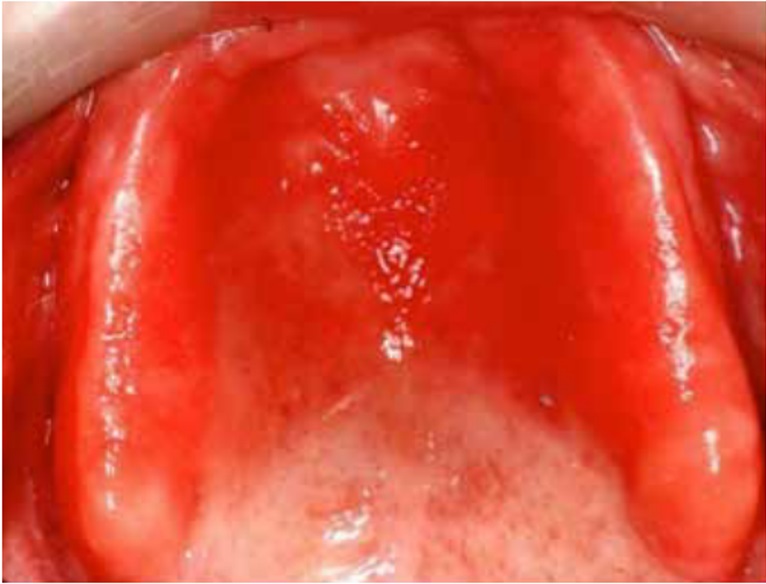
Figure 2: Newton’s Type II DS showing generalized erythema covering the denture-bearing area (13)
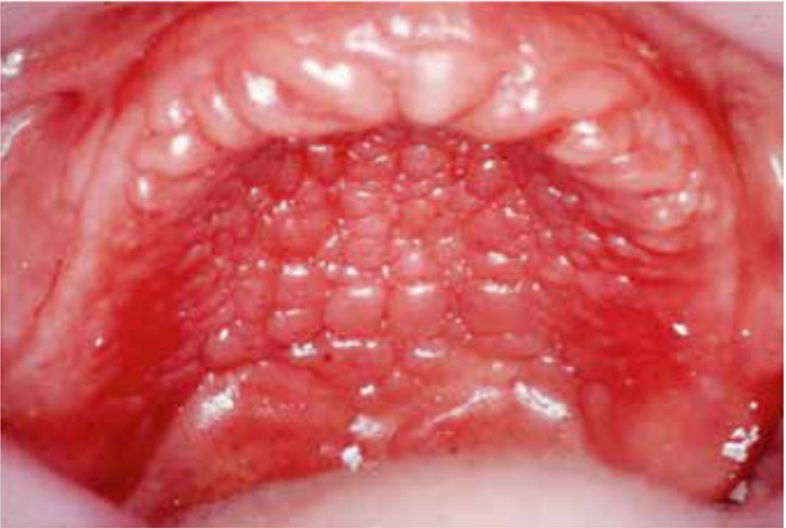
Figure 3: Newton’s Type III DS showing inflammatory papillary hyperplasia (13).
Epidemiology
Candida albicans is the primary strain implicated in the inflammatory pathology of denture stomatitis, although other Candida species like C. dubliniensis, C. parapsilosis, C. krusei, C. tropicalis, and particularly C. glabrata have also been isolated from lesions (11). The development of Candida-associated DS is complex and involves multiple factors. C. albicans, commonly present in the oral cavity, is carried by up to 67% of individuals without causing infection. However, under certain local and systemic conditions, this organism can shift from being harmless to pathogenic. This transition is influenced by the host's immune status. When the host becomes immunocompromised, C. albicans activates and produces hydrolytic enzymes like proteinases and phospholipases. These enzymes aid in the adherence of the fungus to host cells and facilitate the breakdown of cell walls for nutrient absorption, promoting further invasion (12).
Etiology
The causation of DS is complex and involves multiple factors (1, 7, 8). Candida albicans plays a significant role in DS, being implicated in about 90% of cases (13). However, various bacteria like Staphylococcus, Streptococcus, Fusobacterium, and Bacteroides species are also known contributors (14). The transition of Candida from a harmless organism to a harmful one is triggered by an imbalance in the immune relationship between the host and the fungus. This change is often due to weakened host defences and the creation of favorable conditions for Candida growth, leading to tissue irritation (14, 15). Additionally, materials used in dentures, such as acrylic resin, provide an ideal environment for fungal colonization. This material, along with resilient soft linings known for their porous texture, facilitates the adherence and proliferation of fungi.
Predisposing factors
Predisposing factors for DS encompass both systemic and local elements. These include microbial factors, methods of denture cleaning, nocturnal denture wearing, poor fit of the denture, inadequate oral and denture hygiene, xerostomia, smoking habits, the quality and quantity of saliva, occlusal issues, parafunctional habits, carbohydrate-rich diets, age of the denture, and potentially a flaw in the host's immune system (Table 1) (16).
|
Table 1. Summary of predisposing factors for DS, categorized into local and systemic factors. |
||
|
Category |
Factor |
Description/Notes |
|
Local Factors |
||
|
Microorganisms |
Dentures can alter the local environment (pH, saliva flow) and act as a reservoir for microorganisms like Candida albicans, Staphylococcus, Streptococcus, Fusobacterium (17). |
|
|
Trauma |
Ill-fitting dentures can lead to trauma. Incorrect dimensions, unstable dentures and nocturnal wear are risk factors (16). |
|
|
Denture Lining Materials |
Materials like silicone elastomers are prone to Candida colonization, leading to irritation and fungal growth (16). |
|
|
Denture Plaque |
Poor hygiene, high carbohydrate intake, reduced salivary flow, and continuous wearing increase plaque pathogenicity (16). |
|
|
Surface Texture & Permeability |
Microporosities in the denture base facilitate yeast nesting and hinder bacterial elimination (18). |
|
|
Saliva |
Altered saliva composition or flow like in xerostomia can disrupt microbial balance and favor harmful bacteria (19). |
|
|
Systemic Factors |
||
|
Various Conditions |
Diabetes, nutritional deficiencies (iron, folate, vitamin B12), hypothyroidism, immunocompromised states, malignancies, and the use of drugs like corticosteroids increase susceptibility to candida-associated DS (20). |
|
Prevention
Preventing DS is a critical aspect of oral health care, especially for elderly patients. Dental professionals need to educate not only themselves but also caregivers, family members, and patients about effective prevention strategies. The key to this is regular oral examinations to spot early signs of DS, even in the absence of symptoms. Proper care of dentures is essential; this includes thorough cleaning and maintenance of good oral hygiene practices. Patients should be advised to give their gums a rest by removing their dentures for 6–8 hours daily, providing much-needed relief to their oral tissues. For those wearing partial dentures, professional plaque control is vital to prevent DS. These preventative steps, if diligently followed, can significantly reduce the risk and impact of DS, ensuring better oral health and comfort for patients (16).
Treatment
The management of DS, a common condition among denture wearers, requires a multi-faceted approach that encompasses biofilm cleaning, disinfection of the prosthesis, and patient education.
Biofilm cleaning and disinfection at the prosthesis level
The primary treatment of DS targets the cleaning and disinfection of the denture biofilm. A systematic review of the literature suggests that basic mechanical oral and denture hygiene procedures, along with removing dentures during sleep, are among the most effective measures for managing DS (21). For most patients, these simple practices significantly reduce the microbial load and improve oral health. In this case, it is crucial to understand the differences between cleaning, disinfection, and sterilization (22). Cleaning involves the mechanical removal of debris and microbial content, which can be facilitated by brushing with or without the use of a surfactant or dentifrice. Disinfection, in contrast, refers to the killing and destruction of some, but not all, microbial contamination, typically achieved through the immersion of a denture in a chemical microbiocidal solution. Effective disinfection requires prior cleaning since debris and microbial loading can render disinfection procedures ineffective (6). Sterilization, which entails the total elimination of microbial contamination, is not practical for denture care due to the potential damage to the denture base. Therefore, the focus should be on thorough cleaning followed by effective disinfection.
Evidence-based recommendations for denture care
The American College of Prosthodontists has published evidence-based guidelines for the care and maintenance of complete dentures (23). According to these guidelines, dentures should be cleaned daily using a nonabrasive denture cleanser or soap. Care should be taken to avoid exposing dentures to high temperatures or prolonged immersion in sodium hypochlorite solutions, as this can damage the polymethylmethacrylate (PMMA) surfaces (23, 24). Additionally, it is recommended that dentures be removed from the oral cavity during sleep and not worn continuously. When not in use, dentures should be immersed in clean water to prevent warping. Professional cleaning services, such as ultrasonic debridement, should be sought annually to minimize the accumulation of microbial deposits and maintain the polished surfaces of the denture (23).
Denture cleaning techniques
A variety of denture cleaning products, including specific-purpose brushes, creams, and pastes, are available on the market. These products may contain mild abrasives, detergents, fluorosurfactants, and antibacterial agents, depending on their formulation. The use of these dentifrice products has been shown to improve biofilm control compared to brushing with water alone (25). However, they offer similar biofilm control to non-specific cleaning agents like hand soaps or dishwashing liquids (6). Patients should be advised to brush their dentures at least once daily with a soft-bristled brush and very mild abrasive pastes or soaps to remove debris, biofilm, and its byproducts without damaging the denture surfaces.
Chemical disinfection of dentures
Specific chemical disinfectants for dentures are commercially available, mostly based on immersion disinfection processes. These disinfectants may contain active ingredients such as hypochlorites, alkaline peroxides, enzymes, acids, or EDTA. Short-term immersion in 0.5%–2% weight per volume sodium hypochlorite solutions is considered one of the most effective methods for disinfecting dentures (6). However, these solutions can cause corrosion to dentures with metal parts and surface degradation to PMMA prostheses with prolonged use. Therefore, chemical disinfection should be used sparingly and according to the manufacturer's instructions. For dentures with metal frameworks, alkaline peroxide solutions are a viable alternative (26).
Microwave disinfection technique
Another method for denture disinfection is microwave irradiation. This involves immersing the denture in clean tap water and subjecting it to microwave irradiation at a standard kitchen microwave setting. This method has been reported to achieve sterilization, although its long-term effects and potential to cause denture warpage limit its practicality for patient use (23). A Cochrane systematic review assessed various clinical trials on specific denture cleaning and disinfection methods (27). The review found limited evidence to support one method over another, suggesting that simple mechanical brushing and immersion disinfection are generally effective at a prosthesis level.
Antifungal treatment in denture stomatitis
Antifungal treatment can be effective in the short-term management of DS; however, it is not recommended as a first-line therapy due to the likelihood of recurrence in the absence of concurrent improvements in denture hygiene (6). Common antifungal treatments are presented in (Table 2) (6). Despite their effectiveness, antifungal resistance can emerge in patients treated with these medications over short periods (28-32).
|
Table 2. Common antifungal treatments used in managing DS (6). |
||
|
Antifungal Medication |
Dosage and Formulation |
Treatment Protocol |
|
Fluconazole |
50 mg oral tablet |
Once daily; 14 days |
|
Miconazole |
2% Gel applied to denture intaglio |
Three times daily; 14 days |
|
Nystatin |
215,000 IU powder to denture intaglio |
Three times daily; 14 days |
|
Nystatin |
1,000,000 IU mouth rinse/denture soak |
Twice daily; 28 days |
|
Amphotericin B |
10 mg lozenges |
Four times daily; 14 days |
|
Itraconazole |
100 mg oral capsules |
Twice daily; 14 days |
Denture maintenance and replacement prognosis
While the use of older dentures and traumatic denture fit are known exacerbating factors for DS, the fabrication of a new denture does not always guarantee the complete resolution of the condition (33). This underscores the importance of continuous commitment to denture hygiene maintenance by the patient. Annual professional servicing of dentures is recommended to optimize performance and maintain good denture hygiene (23). This servicing can include cleaning, repolishing, relining, and rebasing with high-quality denture base materials. Chairside denture relines materials, often based on auto polymerizing PMMAs, tend to create porous and plaque-retentive surfaces (34, 35). In contrast, laboratory procedures using heat-polymerized PMMAs result in less porous surfaces and offer a higher polish, representing the gold standard in prosthodontics (36). Digitally fabricated milled PMMAs, processed under high pressure and temperature, are increasingly being used clinically due to their superior hygienic and mechanical properties. However, it remains speculative whether these material improvements translate to better resistance against biofilm formation compared to traditional PMMAs. Traditional denture fabrication techniques, including metal frameworks, continue to offer biomechanical and hygienic advantages. For example, the use of thin, lightweight, well-fitting metal frameworks in the maxillary hard palate, the most common site for denture stomatitis, can facilitate denture hygiene procedures (37).
Conclusion
Effective long-term management of DS, a common condition influenced by poor oral and denture hygiene and continuous denture wear, particularly at night, requires a patient-driven approach. This includes daily mechanical cleaning of dentures with a soft brush and mild soap, careful disinfection with suitable solutions like sodium hypochlorite, nightly removal of dentures, and annual professional maintenance, including ultrasonic cleaning and potential relining with quality materials. Proper treatment not only resolves the condition but also reduces health risks, such as aspirational pneumonia, in vulnerable populations.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.