Volume 4, Issue 1
January 2024
Postpartum Pelvic Floor Muscle Training for Reducing Urinary Incontinence: A Systematic Review of Randomized Control Trials
Ahmad Hamad Albegami, Fadwa Hamad Albegami, Faygah Hamad Albegami
DOI: http://dx.doi.org/10.52533/JOHS.2024.40111
Keywords: urine, incontinence, postpartum, PFMT, treatment,systematic review
Postpartum urinary incontinence is an increasingly prevalent problem for women, as it affects about one-third of women. The clinical significance of urine incontinence in relation to deficiencies in the active and passive pelvic floor structures is well recognized. Hence, pelvic floor muscle training (PMFT) treatment for urine incontinence is recommended. Several research studies suggest that PFMT prevents and treats pregnancy- and delivery-related urine incontinence. This study aimed to determine the effectiveness of postpartum PFMT for the treatment of urinary incontinence. Randomized control trials reporting the efficiency of PFMT among postpartum women from 2003–2023 were included. Studies including pregnant women or women who underwent any surgical intervention, along with studies describing pharmacological interventions and others apart from PFMT or PFMT with electrostimulation or biofeedback, were excluded. Additionally, studies with insufficient or unclear data regarding pregnancy outcomes were excluded. A Cochrane bias risk assessment tool was utilized to assess the quality of the studies. Final inclusion resulted in 7 studies that recruited 1398 patients. Four of the included trials assessed the endurance and strength of PFMT, and various rates of strength and endurance were observed among participants in both the intervention and control groups. The prevalence of urinary incontinence in comparison to the control group was observed to be lower in the intervention group, which demonstrates the beneficial role of PFMT in the management of urinary incontinence postpartum. There is strong evidence to suggest the effectiveness of PFMT in the management of postpartum urinary incontinence. However, this beneficial role of PFMT is often overlooked or under-described in clinical practice, for which awareness among clinicians and patients is vital. Additionally, awareness and planning for PFMT execution during antenatal visits can also help prevent urinary incontinence postpartum.
Introduction
Urinary incontinence that occurs after childbirth is an increasingly prevalent problem for women as it affects about one-third of women. Within the first three months after giving birth, 34.3% of women experience various levels of incontinence; 3.3% of these women experience daily urine leaks or greater, and 8.5% of these women require the use of pads. Urine incontinence significantly affects women's quality of life (1, 2). Urinary incontinence refers to any involuntary leakage of urine, encompassing a broad spectrum of manifestations. Stress urinary incontinence, for instance, involves uncontrollable urine leakage during activities like coughing, sneezing, or physical exertion. Urgency urinary incontinence, on the other hand, entails involuntary urine leakage coupled with a sudden, compelling need to urinate. Mixed urinary incontinence refers to involuntary leaking linked to both urgency and effort (3). The most prevalent type of urinary incontinence associated with pregnancy and childbirth is stress urinary incontinence (4).
In the second and third trimesters of pregnancy, over one-third of women develop urinary incontinence, or the unintended loss of urine, and approximately one-third leak urine in the initial three months after giving birth. In late pregnancy, approximately 25% of women experience some involuntary loss of wind or faeces referred to as anal incontinence, and 15% leak wind or faeces a year after giving birth. In addition to being crucial for the people affected, managing incontinence after pregnancy may be highly costly for both the patient and the healthcare system (5). Urinary incontinence affects millions of people globally, with a 9.3% to 67.1% documented prevalence in women. There is robust evidence linking female urine incontinence to pregnancy and vaginal birth. Numerous known risk factors exist for urine incontinence during pregnancy and childbirth, such as the growing uterus's increased abdominal pressure, the fetus's pressure on the pelvic floor muscles, and vaginal delivery-related injury to the pelvic floor muscles' innervation (6).
Numerous healthcare providers have acknowledged the clinical significance of urine incontinence in relation to deficiencies in the active and passive pelvic floor structures, which regulate continence and offer stability in the lumbopelvic area by generating, sustaining, and elevating intra-abdominal pressure (7). While there may be additional reasons linked to the pathologic process of urine incontinence, deficiencies in the pelvic floor muscles have been identified as a significant contributing factor. Thus, improving the strength, endurance, coordination, and contraction speed of the pelvic floor muscles is the theoretical foundation for treating urinary incontinence. This will ensure that the bladder neck is elevated during increased intra-abdominal pressure and that there is adequate urethral closure force (8).
Hence, the first-line treatment for urine incontinence is pelvic floor muscle training (PFMT), which is occasionally combined with electrical stimulation and vaginal cones. In order to prevent urine leakage, the goal of this is to stabilize the bladder neck under abdominal pressure, such as sneezing or coughing. Through levator plate elevation, enhanced pelvic floor muscle growth, and improved surrounding connective tissue, PFMT can also provide continuous support (3). PFMT entails repeatedly performing one or more sets of voluntary pelvic muscular contractions. Depending on the regimen, the exercises vary in terms of frequency, intensity, and progression. More sets of exercises are typically repeated on different days of the week in a normal PFMT program for at least 6–8 weeks. To achieve long-term effects, a maintenance program should be suggested following this initial phase (9, 10).
The use of PFMT in the treatment and prevention of urinary incontinence is justified, considering a number of reasons. By increasing muscle volume, PFMT closes the levator hiatus, shortens the pubovisceral length, raises the bladder's resting position, and elevates the pelvic floor muscle and pelvic organs. All of these anatomical modifications enhance the pelvic floor's structural support (11). Several research studies suggest that PFMT prevents and treats pregnancy- and delivery-related urine incontinence (12). PFMT, both before and after delivery, has been shown to prevent and treat urinary incontinence in studies with sufficient sample sizes, strict adherence to a strength-training regimen, and thorough follow-up. However, uncertainty surrounds the ideal dosage for PFMT to be effective. A training regimen that emphasizes near-maximum contractions and at least an 8-week training period, while adhering to general strength-training principles, can be suggested instead (9).
The guidelines of the National Institute for Health and Care Excellence suggest that during their first pregnancy, women should be offered exercises to strengthen their pelvic floor muscles. Significant damage to the pelvic floor muscles occurs during pregnancy and childbirth, which can occasionally result in postpartum urine incontinence (3). Since urinary incontinence continues to be a significant challenge, especially postpartum, we aim to systematically and comprehensively review the existing literature to gather evidence-based findings for the effectiveness of PFMT in its management. Additionally, we aim to review specifically randomized clinical trials to determine the efficacy of this strategy so that high-quality evidence is presented. Moreover, findings from this study will also provide deep insights and an elaborate understanding of the therapeutic role of PFMT.
Methods
Definition of outcomes and inclusion criteria
Our study aimed to determine the effectiveness of postpartum PFMT for the treatment of urinary incontinence. As a result, we included clinical trials reporting the efficiency of PFMT among postpartum women from 2003–2023. Studies including pregnant women or women who underwent any surgical intervention, along with studies describing pharmacological interventions and others apart from PFMT or PFMT with electrostimulation or biofeedback, were excluded. Additionally, studies with insufficient or unclear data regarding pregnancy were excluded. Moreover, case reports with limited sample sizes and no descriptive statistics were also excluded from this review. Other exclusion criteria were nonhuman or laboratory studies, nonoriginal investigations or incomplete studies, abstract-only articles, protocols, theses, and articles that were not published in English or had no available English information.
Search strategy
Following the successful completion of our intended results, we performed a brief manual screening of potentially included studies to identify relevant keywords for the most appropriate search term. Our search terms included ("Pelvic floor muscle training" OR "pelvic floor muscle exercise" OR "Pelvic floor rehabilitation") AND ("urinary incontinence" OR "urine leakage" OR "Bladder control") AND ("Postpartum" OR "Postnatal" OR "Post-childbirth"). The databases employed for searching included PubMed, Web of Science and Cochrane Library. To ensure the inclusion of all relevant research studies, our search was restricted to the title and abstract of the search results. Once all of our results had been transferred and saved to an Endnote library, we traversed each database to find and eliminate duplicates. Furthermore, we conducted a manual search of the included studies' reference lists, related reviews, and comparable article sections in PubMed to find any papers that our electronic search technique may have overlooked. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines throughout all stages of this systematic review.
Screening and extraction
To ensure the accuracy and quality of our review process, we implemented a double screening strategy, which involved screening both titles/abstracts and full-texts. Two reviewers conducted the screening process in a blinded manner, and a senior member overlooked the entire process and facilitated discussions among the reviewers in case of discrepancies. We constructed an extraction sheet that was organized in a manner relevant to our research objectives, which included baseline characteristics, publication details, abstracts, decisions to include or exclude articles, and the reasons for exclusion. We made sure to include all relevant articles that met our criteria.
Quality assessment
We extracted information from the included studies regarding the potential risk of bias in these studies. To assess the quality of randomized control trial studies, we used the Cochrane bias risk assessment tool (13). It consists of six domains, mostly including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. The tool also assesses others and the overall risk of bias.
Results
Search results
We conducted the search strategies as described above and identified a total of 533 citations, which were then reduced to 80 after removing duplicates. After screening titles and abstracts, only 46 citations were considered eligible for the next steps. Full-text screening narrowed down the number to 7 articles that matched our inclusion and exclusion criteria. Figure 1 shows the detailed search strategy and screening process.
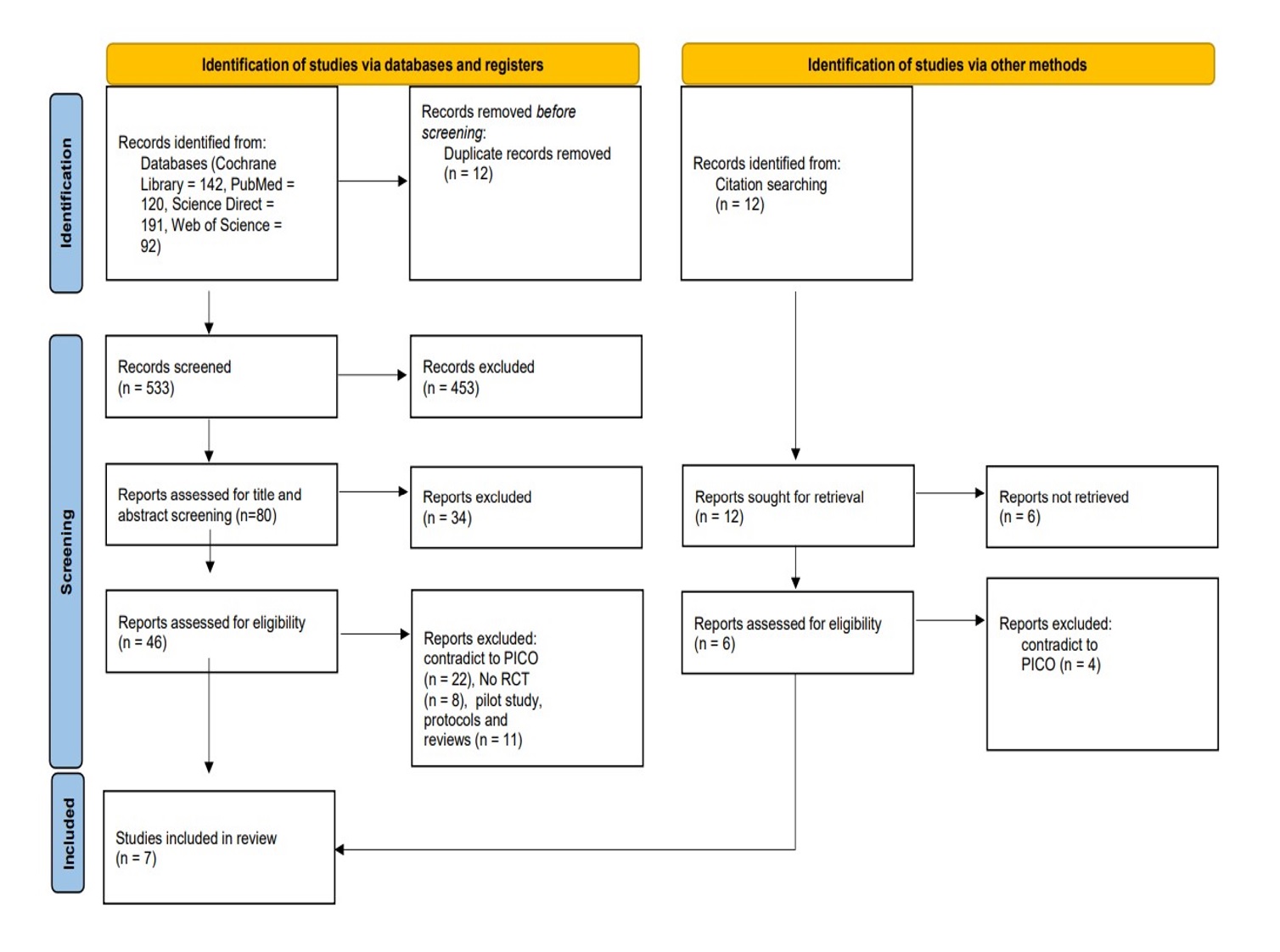
Figure 1: PRISMA flow chart of the included studies
Results of auality assessment
The quality assessment of the included studies revealed that all studies exhibited a low risk of bias, making them high-quality studies. The detailed results of the quality assessment are illustrated in Table 1.
|
Table 1. Evaluation of bias assessment in the included studies using Cochrane bias risk assessment tool |
||||||||
|
Studies |
Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective reporting |
Other bias |
Overall |
|
Sigurdardottir T et al. (14) |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Kaya S et al. (15) |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
Low |
|
Ahlund S et al. (16) |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
Low |
|
Hilde G et al. (17) |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Ko PC et al. (18) |
Low |
Unclear |
Unclear |
Unclear |
Low |
Low |
Low |
Low |
|
Dinc A et al. (19) |
Low |
Low |
Unclear |
Unclear |
Low |
Low |
Low |
Low |
|
Chiarelli P et al. (20) |
Unclear |
Unclear |
Low |
Low |
Low |
High |
Low |
Low |
Characteristics of the included studies
We included 7 studies that recruited 1398 patients and were published between 2004 and 2020 (14-20). The study population consisted of 708 cases and 690 controls in total. All of the included studies were randomized controlled trials. Regarding the geographical distribution of the included studies, two were from Turkey, followed by each study from Norway, Iceland, Australia, China, and Sweden. All the baseline characteristics of these studies are shown in Table 2. There are variations in the sample size of included papers, which are likely due to the objective and inclusion criteria of the specific study.
|
Table 2: Baseline characteristics of the included studies |
||||||||||
|
Author |
Registration |
Country |
Study type |
Year of publication |
Study period |
PFMT (yes/No) |
Total |
Cases |
Control |
Age (Years) (cases/control) |
|
Sigurdardottir T et al. (14) |
NCT02682212 |
Iceland |
RCT |
2020 |
2016 -2018 |
yes |
84 |
41 |
43 |
28 ±4.3/ 29 ±5.3 |
|
Kaya S et al. (15) |
approval no.: 2012/06, LUT 12/36-24 |
Turkey |
RCT |
2014 |
2012 - 2014 |
yes |
108 |
56 |
52 |
48.7±10.1 50.9±8.4 |
|
Ahlund S et al. (16) |
DNR 2009/371-31 |
Sweden |
RCT |
2013 |
NR |
yes |
82 |
40 |
42 |
33 ±3.4/33 ±3.9 |
|
Hilde G et al. (17) |
NCT01069484 |
Norway |
RCT |
2013 |
2010 - 2012 |
yes |
175 |
87 |
88 |
29.5±64.3/ 30.1±64.0 |
|
Ko PC et al. (18) |
No. 96-1224B |
China |
RCT |
2011 |
2008 |
yes |
300 |
150 |
150 |
31.66±3.42/ 31.29±3.78 |
|
Dinc A et al. (19) |
NR |
Turkey |
RCT |
2009 |
NR |
yes |
80 |
40 |
40 |
26.05±4.8 / 27.7±7.2 |
|
Chiarelli P et al. (20) |
NR |
Australia |
RCT |
2004 |
NR |
yes |
569 |
294 |
275 |
Over 35 years |
NR: Not reported; RCT: Randomized controlled trial; PMFT: pelvic floor muscle training.
Study outcome measures
Four of the included trials assessed the endurance and strength of PFMT, among which Sigurdardottir T et al. reported the highest endurance of PFM among intervention and control (234 ±122/ 180 ± 117), respectively, and values observed for PFM strength were 29 ±14 /24 ±13 (14) followed by Kaya et al., who reported a median %(IQR) of 75.5 (54.1-84.7)/ 71.4 (55.4-85.3) among these groups, respectively, for endurance and PFM strength was median (IQR); 3.3(2.3-4.2)/ 2.9(2.1-4.3) (15) while Ahlund et al. stated a median of 26.7 / 23.4 for these groups in the endurance cohort and a median of 4/3 in the strength group (16). Moreover, Hilde et al. observed a mean difference of 27.3 (95% CI: -22.8 to 77.4) in endurance and a mean difference of 3.1 (95% CI: -2.6 to 8.9) in strength (17).
The urinary incontinence prevalence in comparison to the control group was observed to be lower in the intervention group, as reported by Sigurdardottir T et al. (82% and 57%, respectively) (14), while Kaya et al. reported the prevalence among these groups of 76.9%/58.9% (15). However, Hilde et al. and Chiarelli P et al. observed comparable prevalences of 38.6%/34.5% and 36.4%/34.4% among groups, respectively (17, 20). Similarly, in terms of urinary incontinence severity, lower percentages were observed in the intervention group as compared to the control group, as Sigurdardottir T et al. revealed percentages of 27% and 60% for these groups, respectively (14). Kaya et al. reported a baseline median (IQR) of 6.0 (4.0–8.0)/7.0 (4.0–8.7) among observed groups (15). However, Ahlund et al. observed that severity was the same in both groups (median 4) (16). Dinc et al. reported a mean severity of 1.22±0.55/1.79±0.89 among these interventional and control groups, respectively (19). Overall, these findings suggest the positive impact of PFMT on urinary incontinence (Table 3).
|
Table 3. Major Outcomes of the included studies |
||||||||
| Author | UI diagnosis | Group | PFM endurance | PFM strength | PFM Exercise | Prevalence of UI |
UI severity |
UDI-6/IIQ-7 |
|
Sigurdardottir T et al. (14) |
yes |
Intervention/control |
234 ±122/ 180 ± 117, p=.001 |
29 ±14 /24 ±13, p= .003 |
NR |
57%/82% |
27%/ 60% |
NR |
|
Kaya S et al. (15) |
yes |
Intervention/control |
Median%(IQR) 75.5(54.1-84.7)/ 71.4(55.4-85.3) |
Baseline Median (IQR); 3.3(2.3-4.2)/ 2.9(2.1-4.3) |
Median%(IQR) 85 % (75.0–100 %). |
58.9%/ 76.9% |
Baseline Median (IQR): 6.0(4.0-8.0)/ 7.0(4.0-8.7) |
UDI- Baseline Median (IQR): 50.0(33.3-66.6)/ 47.9(30.2-62.5) IIQ- Baseline Median (IQR): 47.6(28.5-66.6)/ 47.6(23.8-66.6) |
|
Ahlund S et al. (16) |
yes |
Intervention/control |
Median: 26.7 / 23.4 |
Median: 4/3 |
NR |
NR |
Median: 4 / 4 |
NR |
|
Hilde G et al. (17) |
yes |
Intervention/control |
Mean difference: 27.3 95% CI: –22.8 to 77.4 |
Mean difference: 3.1 95% CI: –2.6 to 8.9 |
NR |
34.5%/ 38.6% |
NR |
NR |
|
Ko PC et al. (18) |
yes |
Intervention/control |
NR |
NR |
NR |
NR |
NR |
0.35±0.84/ 0.86±1.14 and 0.77±2.07 /1.56±2.20 |
|
Dinc A et al. (19) |
yes |
Intervention/control |
NR |
NR |
NR |
NR |
1.22±0.55 /1.79±0.89 |
NR |
|
Chiarelli P et al. (20) |
yes |
Intervention/control |
NR |
NR |
39.8%/ 32.4% |
34.4%/ 36.4% |
NR |
NR |
UI: urinary incontinence, PFM: Pelvic floor muscle, UDI: Urogenital Distress Inventory, IIQ: Incontinence Impact Questionnaire
Discussion
This study comprehensively analyzed the efficacy and impact of PFMT for the management of urinary incontinence in terms of prevalence and severity. The findings of our review demonstrated that PFMT plays a positive role, as in the intervention group, which included study participants undergoing PFMT, the prevalence and severity of urinary incontinence were reduced. Moreover, various levels of endurance and strength of PFMT were observed among the study participants in both groups.
Similar to our findings, Diz-Teixeira et al. concluded in their review that PFMT is helpful in treating postpartum urine incontinence; however, the authors suggested further home training regimens along with supervised, regulated exercise (21). Results of another systematic review concluded that exercise targeting the pelvic floor muscles is significantly helpful in treating female urinary stress incontinence. Studies have shown that when pelvic floor exercises are performed correctly, incontinence symptoms can improve by up to 70%. Furthermore, authors revealed that research shows that women do better on exercise programs overseen by professional physiotherapists or continence nurses than on self-directed or informational-based programs. The widely held belief that pelvic floor muscle exercise benefits women experiencing all forms of urine incontinence is supported by scientific research (22).
Moreover, another systematic review also highlighted the beneficial role of PFMT, as it concluded that programs for strengthening the pelvic floor muscles during the early and late phases of postpartum recovery have been shown to significantly improve muscular strength and reduce the risk of urine incontinence (23). Findings of another review also showed that supervised intensive programs are more successful than routine postnatal care in the prevention and treatment of postnatal urine incontinence, both immediately after delivery and in cases of persistent incontinence (24).
Over and above, Wang et al. described that in terms of preventing and treating urinary incontinence in postpartum women, several studies have shown that women who get PFMT are less likely than those who do not report urinary incontinence following delivery. Additionally, it has also been shown by earlier systematic reviews that PFMT improves urinary incontinence in postpartum and pregnant women (25). Furthermore Boyle et al. reported in their review findings that women who underwent PFMT and had persistent urine incontinence three months after delivery were found to have a reduced likelihood of reporting urinary incontinence twelve months later compared to those who did not receive treatment or received standard postnatal care (approximately 40% less; risk ratio 0.60, 95% CI 0.35 to 1.03). It appeared that the treatment effect increased with program intensity (26). Findings of a meta-analysis from present times also stated that urinary incontinence treated with individual PFMT during the postnatal period was of clinical significance and cost-effective (27).
Similarly, findings from another review of the current time concluded that urinary incontinence was reduced and pelvic floor muscle contraction was enhanced by PFMT, either by itself or in combination with biofeedback or electrostimulation. While there were no notable differences between PFMT and other interventions, including biofeedback, vaginal cones, and whole-body vibration training, it was still better than the control group (28). Results of a study by Haddow et al. suggest that PFMT can help with postpartum urine incontinence and that performing any form of targeted pelvic floor muscle exercise program seems to increase the frequency of exercise. However, less is known about the benefits of specific elements of pelvic floor muscle exercise programs, such as take-home materials, phone calls serving as reminders, and feedback on how well exercises are working (29).
Kegel originally reported the use of PFMT in 1948 to treat symptomatic women's urine incontinence. The five steps of PFMT procedures are typically informational, posture correction, awareness stage and proprioceptive neuromuscular facilitation, strengthening programs, and perineal blockade before stress. All five stages are crucial and consequential. Three key factors that significantly affect the quality of the evidence and enhance the heterogeneity of the results are when to begin, how to conduct it, and to whom PFMT should be administered (11). Similarly, in our study, various patterns and values for the strength for PFMT were observed, which clearly define that till now there is no standardized practice or guidelines for the schedule and strength of PFMT.
Grant et al. narrated that due to the pelvic floor's straining and injury during childbirth, there is a significant risk of urine incontinence. Therefore, in order to delay the onset of urine incontinence, PFMT is frequently advised during pregnancy. But during their prenatal care, women are inundated with health messages; PFMT is frequently overlooked as a priority, and in the absence of sufficient assistance, women feel helpless, which lowers their participation and self-efficacy. An elevated body mass index, pregnancy, childbirth, and age are all risk factors for urine incontinence. This implies that postnatal interventions may be used to treat or prevent urine incontinence. However, it is unclear if a postnatal population-based strategy for administering PFMT is appropriate or successful in preventing or lessening urine incontinence in the longer term (30).
Moreover, Romeikien? KE et al. described that results have shown that antenatal PFMT lowers the incidence of postpartum urine incontinence and helps avoid it in late pregnancy. The effects of preventative PFMT are long-lasting. The greatest outcomes are achieved by continent women who begin organized PFMT early in their pregnancies. This symptom has been evaluated in the greatest number of studies, and the quality of the evidence is highest here. Prenatal and postnatal PFMT have the potential to enhance quality of life, lessen postpartum urogenital distress, and lessen postpartum urine symptoms. It should be provided to patients in writing instructions on how to perform PFMT (31). Urinary and fecal incontinence have been treated with a variety of approaches, including conservative physical therapy such as PFMT, behavioral training, lifestyle changes, anti-incontinence devices, medication, and surgery. Of these, PFMT is more frequently employed during a woman's pregnancy or the postoperative period than pharmacological or surgical intervention. Training the pelvic floor muscles entails repeatedly contracting them, which enhances muscle tone and strengthens the pelvic floor muscles and perineal support. Strengthening the peri-vaginal and perianal musculature attempts to give women more control over urine flow. Thorough scientific research indicates that PFMT is still the recommended initial course of treatment for female urinary incontinence and that it works well as a preventative measure for urinary incontinence for 6 months postpartum (4).
The majority of the studies in the literature are in accordance with the results of our study and highlight the beneficial role of PFMT for urinary incontinence management postpartum. However, contrary to the findings, Soave et al. narrated that the effectiveness of PFMT in treating and preventing urinary incontinence throughout pregnancy and the postpartum period is currently unproven due to insufficient evidence. Although the authors further agreed that the evidence from the large sample size, clearly defined training protocols, high adherence rates, and close follow-up studies suggests that a PFMT program based on general strength-training principles can be advised for both the prenatal and postnatal phases of pregnancy (11). Similarly, Yang X et al. concluded that there was insufficient data to support the efficacy of group-based PFMT during the postnatal phase (6). This necessitates the need for further evidence-based research, especially meta-analysis and systematic reviews, so more robust conclusive findings are available. Our review delves deeply into assessing the impact of PFMT, specifically on postpartum urinary incontinence. The systematic search methodology and the analysis of all keywords in this field are the strengths of our study. Moreover, the inclusion of all randomized control trial studies with a low risk of bias adds to the advantages and strengths of this study since the evidence and findings presented are from high-quality studies. However, our study has certain limitations. First, the inclusion of only seven trial studies may limit the generalizability of our findings. Secondly, we could not assess the efficacy of PFMT in the longer term as one of the included trials was a follow-up study, and follow-ups for other trials are lacking; therefore, future research studies with follow-ups are needed to back up the efficacious role of PFMT in the treatment of postpartum urinary incontinence.
Conclusion
There is strong evidence to suggest the effectiveness of PFMT in the management of postpartum urinary incontinence since decreased prevalence and severity of urinary incontinence were observed in postpartum women who underwent PFMT however this beneficial role of PFMT is often overlooked or under described in the clinical practice for which awareness among clinicians and patients is vital therefore further research in this aspect is need of time as it can further signify the critical role of PFMT additionally awareness and planning for the PFMT execution during antenatal visits can also help in preventing urinary incontinence postpartum leading to reduction in the overall burden of this complication.
Disclosure
Conflict of interest
There is no conflict of interest
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.
