Volume 4, Issue 1
January 2024
A Case of Non-Immune Mediated Hemolysis Associated with COVID-19 Infection and Review of Literature
Feras Al-Fararjeh, Abdullah Saleh Al-Abbadi, Tariq Nazih Al-Adily, Bashayer Sadiq Abdulrasoul, Sarah Sayed Al-Razouqi, Zahra Muneer Yusuf
DOI: http://dx.doi.org/10.52533/JOHS.2024.40110
Keywords: COVID-19, Direct Coombs test, hemoglobin, non-immune hemolytic anemia
Background: COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), presents a broad spectrum of clinical manifestations, ranging from mild symptoms to severe conditions requiring intensive care. Beyond its well-known respiratory complications, COVID-19 has been increasingly associated with various immune and hematological complications, including cases of hemolytic anemia. This report adds to the emerging evidence linking COVID-19 to hematological abnormalities, specifically non-immune mediated hemolytic anemia (NIHA).
Case Presentation: We report a case of NIHA associated with COVID-19 infection. A 35-year-old male nurse, with no prior health issues or medication allergies, presented with symptoms of generalized weakness, fever, exertional dyspnea, heart palpitations, and dark urine, leading to a COVID-19 diagnosis. Initial examinations revealed fever and jaundice, but normal chest x-ray and oxygen saturation levels. Hematological investigations indicated hemolytic anemia, with a negative direct Coombs test and normal G6PD levels, excluding autoimmune causes and G6PD deficiency. Following treatment with intravenous fluids, paracetamol, and dexamethasone, his symptoms rapidly improved, and subsequent tests three months later confirmed the resolution of hemolysis and normalization of hemoglobin levels.
Conclusion: In COVID-19 infections, various hematological complications can lead to hemolysis, often associated with factors like G6PD deficiency, specific medications, or autoimmune reactions. This report details a unique case of non-immune mediated hemolysis in a patient exhibiting symptomatic COVID-19, notably in the absence of these common hemolytic triggers. The case highlights the need for heightened awareness of atypical hematological presentations in COVID-19 patients.
Introduction
The novel coronavirus disease, COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as a global health crisis of unprecedented scale. Characterized by its highly contagious nature, COVID-19's clinical presentation spans a wide spectrum, ranging from asymptomatic cases to critical conditions necessitating mechanical ventilation (1). The diversity in the severity of symptoms, including fever, cough, fatigue, muscle aches, and more severe respiratory distress, reflects the virus's complex pathophysiology.
During the pandemic, the medical community gained significant insights into the multifaceted nature of COVID-19. Beyond the respiratory system, the virus has demonstrated a capacity to induce systemic effects, particularly in the immune system (2). An intense immune response triggered by the virus can lead to immunological dysregulation and systemic inflammation, often culminating in a pro-thrombotic state. This hypercoagulable condition predisposes patients to a range of severe complications such as respiratory failure, sepsis, and pneumonia, demanding intensive medical intervention (3, 4).
A critical area of ongoing research is the association of COVID-19 with hematological abnormalities. Hemolytic anemia, characterized by the rapid destruction of red blood cells, has emerged as a noteworthy complication in COVID-19 patients (5). This condition, presenting symptoms like fatigue, weakness, and jaundice, adds complexity to the management of COVID-19 due to its diverse etiology. While cases of hemolytic anemia linked to factors such as glucose-6-phosphate dehydrogenase (G6PD) deficiency and medication-induced hemolysis are documented (6), the pathogenesis of COVID-19 induced hemolytic anemia, particularly of the non-immune type, remains less understood. It is postulated that the virus’s direct impact on the hematological system or its secondary inflammatory response may play a crucial role in the onset of this condition. In this case report, we detail the occurrence of non-immune mediated hemolytic anemia in a young male patient following a COVID-19 infection, contributing to the growing body of evidence that suggests a broader impact of SARS-CoV-2 on hematological functions.
Case Presentation
A 35-year-old male, previously in good health and with no known medication allergies, presented to the emergency department on November 15, 2020. He reported experiencing generalized weakness, fever, shortness of breath during exertion, and heart palpitations for one day, along with dark urine. He denied any recent changes in diet or medication use and stated no family history of hematological diseases. There were no prior episodes of similar symptoms. Upon examination, he was found to have a fever (oral temperature of 38.2°C), and displayed signs of jaundice, evidenced by a yellowish discoloration of the skin and sclera. His chest x-ray and oxygen saturation levels were normal. He is employed as a nurse at a COVID-19 treatment center and was on duty when his symptoms began. A nasopharyngeal PCR test conducted on November 18, 2020, confirmed he was positive for COVID-19.
Initial hematological investigations revealed findings consistent with hemolytic anemia and a negative direct Coombs test. To further evaluate the cause of the hemolysis, Hemoglobin electrophoresis was performed to rule out hemoglobinopathies, and the results were normal. Additionally, his G6PD levels were assessed to exclude G6PD deficiency as a cause of hemolysis. The G6PD tests, conducted three weeks and two months post-presentation, both returned normal results. Table 1 summarizes the blood investigation findings. A blood smear taken at the time of presentation indicated hemolysis and is shown in Figure 1. Additional imaging studies, including chest and abdominal X-rays, abdominal ultrasound, and CT scans of the chest, abdomen, and pelvis, showed no abnormalities in lymph nodes, liver, or spleen.
|
Table 1. Initial Laboratory Blood Test Results at Presentation |
||
|
Test |
Result |
Reference Values |
|
RBS (Random Blood Sugar) |
117 mg/dL |
70-99 mg/dL |
|
K (Potassium) |
4.6 mmol/L |
3.5-5.0 mmol/L |
|
Urea |
39 mg/dL |
7-20 mg/dL |
|
Creatinine |
0.47 μmol/L |
0.84-1.21 μmol/L |
|
Bilirubin Total |
5.5 mg/dL |
0.1-1.2 mg/dL |
|
Bilirubin Direct |
0.8 mg/dL |
0.0-0.3 mg/dL |
|
Total Protein |
7.0 g/dL |
6.4-8.3 g/dL |
|
Albumin |
4.5 g/dL |
3.5-5.5 g/dL |
|
ALT (Alanine transaminase) |
18 IU/L |
7-56 IU/L |
|
AST (Aspartate Aminotransferase) |
38 IU/L |
10-40 IU/L |
|
GGT (Gamma-Glutamyl Transpeptidase) |
14 units/L |
9-48 units/L |
|
ALK (Alkaline Phosphatase) |
66 IU/L |
44-147 IU/L |
|
LDH (Lactate Dehydrogenase) |
1653 U/L |
135-225 U/L |
|
COVID 19-SARS |
Positive |
- |
|
Vit B12 |
362 |
200-900 pg/mL |
|
Ferritin |
2443.6 ng/mL |
30-400 ng/mL |
|
Hb (Hemoglobin) |
10.3 g/dL |
13.8-17.2 g/dL |
|
WBC (White Blood Cells) |
16.2 x 10^9/L |
4.0-11.0 x 10^9/L |
|
Platelets count |
213 x 10^9/L |
150-450 x 10^9/L |
|
Reticulocyte count |
8.18 x 10^9/L |
0.5-2.5 x 10^9/L |
|
ESR (Erythrocyte Sedimentation Rate???????) |
9 mm/h |
0-22 mm/h |
|
PT (Prothrombin Time) |
15.2/13.3 |
9.5-13.5 seconds |
|
PTT (Partial Thromboplastin Time) |
24.7/30 |
25-35 seconds |
|
INR (International Normalised Ratio) |
1.16 |
0.8-1.2 |
|
G6PD |
Normal |
4.6 – 13.5 unit/g |
|
Haptoglobin |
0.79 g/L |
0.3-2.0 g/L |
|
Direct Coombs |
Negative |
- |
|
Indirect Coombs |
Negative |
- |
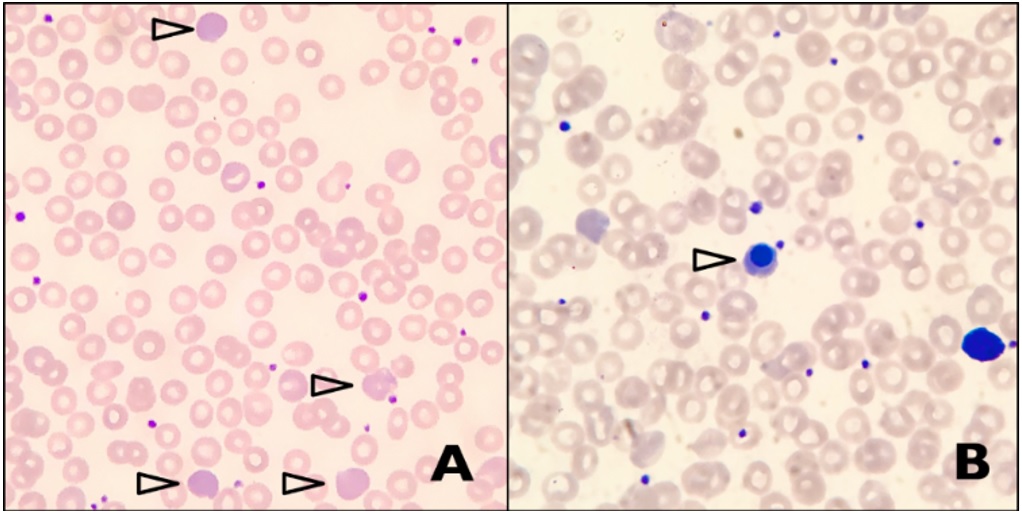
Figure 1: A: Peripheral blood smear shows predominantly normochromic normocytic red blood cells, with prominent polychromasia (arrowheads). Other features of hemolysis such as schistocytes, spherocytes, sickle cells and agglutinated red cells are absent. B: Occasional circulating nucleated red blood cells are noted (arrowhead).
The patient was administered intravenous fluids, discharged with advice to take paracetamol, and self-administered a single 8 mg dose of dexamethasone the following day. His symptoms, including jaundice and red urine, improved within two days of initial presentation. Three months later, repeat testing showed a resolution of the hemolysis and normalization of hemoglobin levels. Repeated blood investigations are summarized in Table 2.
|
Table 2. Follow-Up Laboratory Blood Test Results |
||
|
Test |
Result |
Reference Values |
|
Urea |
25.9 mg/dL |
7-20 mg/dL |
|
Creatinine |
0.59 μmol/L |
0.84-1.21 μmol/L |
|
Bilirubin Total |
0.45 mg/dL |
0.1-1.2 mg/dL |
|
Bilirubin Direct |
0.157 mg/dL |
0.0-0.3 mg/dL |
|
LDH |
364 U/L |
135-225 U/L |
|
Hb |
15.4 g/dL |
13.8-17.2 g/dL |
|
Reticulocyte count |
1.97 x 10^9/L |
0.5-2.5 x 10^9/L |
|
G6PD |
Normal |
4.6 – 13.5 unit/gm |
Literature Review
The association between COVID-19 and various hematological abnormalities, including coagulopathy and disseminated intravascular coagulation (DIC), has been well documented. However, the occurrence of hemolysis in the absence of DIC in COVID-19 patients remains less frequently reported and is an area of ongoing research. The exact mechanisms through which SARS-CoV-2, the virus causing COVID-19, induces hemolysis are not yet fully understood.
In other viral infections, the production of reactive oxygen and nitrogen species has been observed. These reactive species can cause damage to intracellular proteins and DNA, especially in cells with compromised antioxidant enzyme systems (7). This process could potentially be a contributing factor in the pathogenesis of hemolysis associated with COVID-19.
One significant aspect of COVID-19-associated hemolytic conditions is autoimmune hemolysis. This condition occurs when the immune system mistakenly attacks and destroys red blood cells. Research indicates an increased prevalence of anemia among hospitalized COVID-19 patients (8-11). A specific study revealed that 12.7% of hospitalized anemic patients with COVID-19 had a positive direct Coombs test (indicative of autoimmune hemolysis), after ruling out other interfering factors like recent blood transfusions, autoimmune diseases, or lymphoproliferative disorders (10). Lazarian et al. (2020) documented seven cases (ages ranging from 61 to 89 years) with various comorbidities including hypertension, renal failure, and diabetes (11). These patients presented with mild to severe COVID-19 symptoms. Notably, some had underlying hematological disorders like chronic lymphocytic leukemia (CLL) and marginal zone lymphoma (MZL). All tested positive on the Direct Coombs test and did not receive hydroxychloroquine.
In addition to the well-documented coagulopathies and autoimmune hemolysis associated with COVID-19, there's a growing recognition of nonimmune hemolytic anemia linked to the infection (12). This form of anemia arises from mechanisms unrelated to the immune system's attack on red blood cells. The pathophysiology behind this condition in the context of COVID-19 is multifaceted and not entirely understood. It is hypothesized that the virus may directly affect the bone marrow's red blood cell precursors or cause indirect damage through increased oxidative stress, especially in individuals with underlying conditions like G6PD deficiency. G6PD deficiency is an X-linked genetic disorder that reduces the effectiveness of red blood cells to cope with oxidative stress, leading to their premature destruction (hemolysis) under certain conditions (13). Several case reports have highlighted acute hemolytic anemia in patients with concurrent G6PD deficiency and COVID-19 infection (14-23). For instance, Lopes et al. described a case involving a 35-year-old male without prior comorbidities, diagnosed with G6PD deficiency five months following an episode of hemolytic anemia linked to COVID-19 (23). Similarly, another report detailed the case of a 62-year-old male with a history of type 2 diabetes and hypertension, who presented with acute hemolysis alongside COVID-19 pneumonitis and was later found to have G6PD deficiency during his hospital stay. (22)
The use of hydroxychloroquine, initially explored as a treatment for COVID-19, raises additional concerns for patients with G6PD deficiency. Hydroxychloroquine is known to potentially exacerbate hemolysis in such patients. However, a study involving 11 patients with G6PD deficiency who were administered hydroxychloroquine reported no episodes of hemolysis, suggesting a degree of safety in its use (24). Nonetheless, the safety of higher doses of hydroxychloroquine in this context remains uncertain and warrants caution.
Additionally, the widespread clotting seen in severe COVID-19 cases can lead to mechanical destruction of red blood cells in microvascular thromboses (25). Furthermore, severe respiratory symptoms and the associated systemic hypoxia may place additional stress on red blood cells, exacerbating hemolysis(26). This hyperinflammatory state, a hallmark of severe COVID-19, could also indirectly contribute to red blood cell damage. Clinically, nonimmune hemolytic anemia in COVID-19 patients might be subtle and is diagnosed based on laboratory findings such as elevated lactate dehydrogenase (LDH), decreased haptoglobin, and increased indirect bilirubin, along with blood smear analysis. Differentiating this condition from other forms of anemia is critical, as it necessitates specific management strategies focusing on supportive care and treating the underlying COVID-19 infection while addressing any oxygenation issues. This expanding understanding of COVID-19's hematological impact highlights the need for thorough diagnostic evaluations and individualized treatment plans for affected patients. Heightened awareness and careful monitoring of hemolytic conditions in COVID-19 patients, especially those with pre-existing conditions like G6PD deficiency or a predisposition to autoimmune responses is, therefore, crucial. Further research is essential to unravel the mechanisms behind these associations and to guide optimal management strategies for affected patients.
Discussion
The presented case of a 35-year-old male with no significant medical history, developing non-immune mediated hemolytic anemia in the context of COVID-19, provides insight into the lesser-known hematological manifestations of SARS-CoV-2 infection. This case is particularly intriguing due to the absence of common precipitating factors for hemolysis and reveals the need for more attention to the hematological impacts of COVID-19. The patient's clinical presentation, including generalized weakness, fever, exertional dyspnea, and palpitations, coupled with laboratory findings indicative of hemolysis (such as elevated LDH levels and abnormal RBC indices), pointed towards an acute hemolytic process. The rapid onset of symptoms following exposure to COVID-19 as a frontline healthcare worker adds another layer of complexity to the case, suggesting a possible direct viral effect or a secondary response to the infection leading to hemolysis.
A key aspect of this case is the exclusion of autoimmune causes, evidenced by negative Direct and Indirect Coombs tests. Furthermore, the normal results for G6PD levels and hemoglobin electrophoresis ruled out other common etiologies of non-immune mediated hemolysis. These findings are critical in reinforcing the likelihood that the patient's hemolytic anemia was a direct consequence of the COVID-19 infection. Such instances, though rare, suggest that COVID-19 might have a direct pathogenic role in inducing hemolysis, possibly through mechanisms like direct viral invasion of erythroid precursors, induction of oxidative stress on erythrocytes, or triggering a hyperinflammatory state that leads to increased erythrocyte fragility and destruction (27).
The patient's rapid recovery following the administration of intravenous fluids and a single dose of dexamethasone raises interesting considerations about the therapeutic approach in such cases. While dexamethasone is known for its efficacy in severe COVID-19 due to its anti-inflammatory properties (28), its role in directly influencing the course of hemolytic anemia in COVID-19 patients is not well established and merits further investigation. This improvement also brings to light the self-limiting nature of the hemolytic process in some COVID-19 cases, where supportive care can suffice without the need for aggressive interventions.
Additionally, this case emphasizes the importance of vigilant monitoring for atypical presentations of COVID-19. It enriches our understanding of the virus's hematological effects, particularly considering the absence of usual precipitating factors for hemolysis. This scenario is mirrored in the broader landscape of COVID-19-related hematological complications, as detailed in various studies. For instance, Beauverd et al. (2020) reported a pediatric case showing symptomatic COVID-19 with a background of hereditary spherocytosis and sickle cell trait, shedding light on the potential for COVID-19 to exacerbate pre-existing hematological conditions (18). Further, Lancman et al. investigated the occurrence of Coombs-negative hemolytic anemia in COVID-19 patients, prompted by declining hemoglobin levels and the potential masking of hemolysis by universally elevated lactate dehydrogenase (LDH) and acute phase haptoglobin (12). The study, which involved testing 38 patients for hemolysis and plasma hemoglobin (plasma-Hb) levels, found that 82% of the patients had elevated plasma-Hb, a marker of intravascular hemolysis known to be pathogenic in various conditions including acute respiratory distress syndrome (ARDS). Notably, 24% of patients had highly pathogenic plasma-Hb levels (> 30 mg/dL), and a majority of these patients exhibited elevated LDH, reticulocytosis, low haptoglobin, and elevated bilirubin, though peripheral blood smears showed minimal schistocytosis. Their study reiterates that intravascular hemolysis may be an under-recognized complication in COVID-19, with a high prevalence of elevated plasma-Hb even in the presence of elevated haptoglobin. This finding is significant as it proposes a potential link between plasma-Hb and the pathophysiology of COVID-19’s hematological complications. Therefore, healthcare professionals should be aware of this aspect of the disease in patients with COVID-19, even in the absence of pre-existing hematological disorders or other identifiable causes of hemolysis, like in our case. Early detection and management of such complications could play a crucial role in improving patient outcomes. Summary of reported cases from the literature involving patients with hemolysis associated with COVID-19 infection is depicted in Table 3.
|
Table 3. Summary of reported cases of patients with hemolysis associated with COVID-19 infection |
|||||||
|
Study |
Age |
Comorbidity |
COVID-19 |
Underlying hematological disease |
Use of hydroxychloroquine |
Direct Coombs |
|
|
Non-immune hemolysis |
Lopes et al., 2020 (23) |
35 |
NA |
Symptomatic |
G6PD deficiency |
No |
Negative |
|
Palmer et al., 2020 (22) |
62 |
Diabetes mellitus type 2, Hypertension |
Pneumonitis |
G6PD deficiency |
No |
Negative |
|
|
Naymagon et al., 2020 (21) |
50 |
NA |
Acute hypoxic Respiratory failure due to COVID-19 |
NA |
Yes |
NA |
|
|
Naymagon et al., 2020 (21) |
52 |
Obesity, Diabetes mellitus |
Acute Hypoxic respiratory failure with COVID-19 |
NA |
Yes |
NA |
|
|
Naymagon et al., 2020 (21) |
54 |
Diabetes mellitus |
Acute hypoxic Respiratory failure due to COVID-19 |
G6PD deficiency |
Yes |
Negative |
|
|
Kuipers et al., 2020 (19) |
56 |
Diabetes mellitus |
Symptomatic |
G6PD deficiency |
No |
NA |
|
|
Beauverd et al., 2020 (18) |
68 |
Diabetes type 2, Hypertension, Chronic renal insufficiency (G2 stage), History of stroke |
Symptomatic |
G6PD deficiency |
Yes |
NA |
|
|
Severance et al., 2020 (17) |
4 |
NA |
Symptomatic |
Moderate hereditary spherocytosis, Sickle cell trait |
No |
NA |
|
|
Maillart et al., 2020 (16) |
65 |
Hypertension, Diabetes type 2 |
Acute respiratory distress syndrome |
G6PD deficiency |
Yes |
NA |
|
|
Sasi, Yassin, Nair, & Al Maslamani, 2020 (15) |
26 |
none |
Pneumonia |
Asymptomatic hemoglobin D thalassemia, G6PD deficiency |
Yes |
NA |
|
|
Aguilar & Averbukh, 2020 (14) |
51 |
Hypertension, Diabetes type 2, Obesity |
Pneumonia |
G6PD deficiency |
Yes |
NA |
|
|
Autoimmune hemolysis |
Lazarian et al., 2020 (11) |
61 |
Hypertension, chronic renal failure |
Moderate |
Chronic lymphocytic leukaemia |
No |
Positive |
|
Lazarian et al., 2020 (11) |
89 |
Hypertension, chronic renal failure, atrial fibrillation |
Mild |
Monoclonal gammopathy of undetermined significance |
No |
Positive |
|
|
Lazarian et al., 2020 (11) |
62 |
Hypertension, cirrhosis |
Severe |
Marginal zone lymphoma |
No |
Positive |
|
|
Lazarian et al., 2020 (11) |
69 |
Obesity |
Moderate |
Marginal zone lymphoma |
No |
Positive |
|
|
Lazarian et al., 2020 (11) |
61 |
Hypertension, chronic renal failure, diabetes, hypercholesterolaemia |
Mild |
none |
No |
Positive |
|
|
Lazarian et al., 2020 (10) |
61 |
Diabetes |
Severe |
none |
No |
Positive |
|
|
Lazarian et al., 2020 (11) |
75 |
Diabetes, hypercholesterolaemia, cardiopathy, obesity, chronic obstructive bronchopneumopathy |
Moderate |
Chronic lymphocytic leukaemia |
No |
Positive |
|
|
Jacobs & Eichbaum, 2021 |
33 |
history of hypothyroidism |
Symptomatic |
none |
No |
Positive |
|
Conclusion
This case of non-immune mediated hemolytic anemia following a COVID-19 infection significantly contributes to our understanding of the hematological manifestations of SARS-CoV-2. The absence of typical causes of hemolysis, alongside negative Coombs tests and normal G6PD levels, strongly suggests a direct link between COVID-19 and the onset of hemolytic anemia. Such cases reveal the virus’s potential for directly affecting erythrocytes as well as for triggering secondary hyperinflammatory responses leading to hemolysis. The rapid resolution of symptoms with supportive care, in this case, suggests a potential role for anti-inflammatory treatment in managing such hematological complications of COVID-19. This report thereby reinforces the necessity for heightened clinical vigilance and comprehensive diagnostic approaches in COVID-19 patients, particularly as we continue to unravel the full spectrum of the virus's impact on human health.
Disclosure
Conflict of interest
There is no conflict of interest
Funding
No funding
Ethical consideration
A written informed consent was taken from the patient to include the data in the form of case publication with protection of the patient’s anonymity.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, clinical data drafting, diagnosis, treatment and final writing of the manuscript.
