Volume 3, Issue 12
December 2023
Neuropathic Pain Management, Pharmacological Approaches, Neuromodulation and Quality of Life
Bayan Ali, Reem Alqubaishi, Fahad Asiri, Mohammed Alkhadhrawi, Alhanouf Aldhohayan, Faisal Aljabri, Abdullah Alahmari, Rahaf Alharbi, Meshal Altowairqi, Raed Alshawaf, Khalid Alzahrani
DOI: http://dx.doi.org/10.52533/JOHS.2023.31232
Keywords: neuropathic, pain, pharmacological, therapy, quality of life
Neuropathic pain, which affects 7%–10% of the general population, is caused by a lesion or disease of the somatosensory system, which includes peripheral fibers and central neurons. There are numerous known causes of neuropathic pain, and as the world's population ages, the prevalence of diabetes mellitus rises, and cancer patients are more likely to survive post-chemotherapy. Its occurrence is projected to rise. The intricacy of neuropathic symptoms, adverse outcomes, and challenging treatment choices all appear to contribute to the burden of chronic neuropathic pain. Crucially, neuropathic pain patients have a lower quality of life due to a higher need for prescription drugs, more frequent visits to the physician, the morbidity from the pain itself, and the underlying disease. The most effective strategy to treat neuropathic pain would employ the right combination of pharmacological and nonpharmacologic therapy. As first-line treatments, gabapentinoids, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors are advised while tramadol and opioids are regarded as second-line therapy. Because there are currently insufficient high-quality trials, cannabinoids are advised as third-line treatments, and methadone and some anticonvulsants are suggested as fourth-line therapies. In cases of failure of pharmacological therapy or other special considerations, neuromodulation therapy is suggested, which involves deep brain stimulation, spinal cord stimulation, transcranial magnetic stimulation, and transcranial current stimulation and is broadly categorized under invasive and non-invasive neuromodulation procedures. In this study, we aim to comprehensively review the existing management strategies for neuropathic pain, specifically in the context of pharmacological, neuromodulation, and quality of life domains.
Introduction
Chronic pain is the leading cause of disability globally, when all forms of pain are taken into consideration, including low back pain and headache disorders. Chronic pain is typically described as pain that lasts for three months or more (1). Chronic pain is considered a global public health concern by the World Health Organization due to its significant impact on both individuals and society (2). The more severe form of chronic pain is neuropathic pain (NP) (1). In the general population, NP is prevalent, since between 7% and 10% of adults report having persistent NP (3).
NP refers to a condition that develops from a primary lesion or disease of the somatosensory nerve system. This disorder is characterized by a number of distinct pathogenic mechanisms. The pathophysiological states and conditions that determine the onset of NP are primarily a result of metabolic disorders, such as peripheral diabetic neuropathy, neuropathies linked to viral infections, such as post-herpetic neuralgia, HIV, and leprosy, autoimmune disorders affecting the central nervous system, such as multiple sclerosis and Guillain-Barre syndrome, peripheral neuropathies induced by chemotherapy, damage to the nervous system resulting from traumatic events, such as spinal cord injury and amputation, inflammatory disorders, hereditary neuropathies, and channelopathies (4).
There is a correlation between NP and increased prescription drug usage and visits to the physician. Patients usually present with a specific combination of symptoms, and these symptoms tend to be persistent, chronic, and less responsive to analgesics. Patients experiencing NP frequently have severe sleep disturbances, anxiety, and depression. Additionally, quality of life is more compromised in NP patients as compared to non-NP patients (5). Similar to other pain syndromes, the objectives of treating NP include reducing pain, improving function and quality of life. The most effective strategy to treat NP would be to use a whole-person (biological, psychological, social, and spiritual) perspective, utilize a multidisciplinary approach, prevent or treat any underlying causes, and employ the right combination of pharmacological and nonpharmacologic therapy. As first-line treatments, gabapentinoids, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors are advised. Due to their propensity for adverse effects, abuse, and medical complications, as well as the difficulty of their follow-up and monitoring requirements, tramadol and opioids are regarded as second-line therapies. Because there are currently insufficient high-quality trials, cannabinoids are advised as third-line treatments. Methadone and tapentadol, a less effective anticonvulsant, are suggested fourth-line therapies (6).
However, pharmacological drugs can cause major adverse reactions, including addiction, overdose, and even death, and they do not have a long-lasting impact on reducing pain. When pharmacological treatments are ineffective for a patient, invasive neuromodulation procedures, including deep brain stimulation, motor cortex stimulation, and spinal cord stimulation, are used. Invasive stimulations, on the other hand, are very expensive, require accurate target site determination, and carry a risk of infection and other repercussions. New techniques for treating persistent NP have emerged as a result of recent developments in non-invasive neuromodulation. Though they have limited deep penetration and spatial focality, modern non-invasive stimulation techniques like transcranial magnetic stimulation and transcranial current stimulation have demonstrated potential pain reduction results (7).
The quality of life can be greatly impacted by NP, which can be severe, persistent, and debilitating. Researching and developing effective management strategies can alleviate pain and enhance the overall well-being of individuals suffering from NP. Moreover, studying different management strategies can lead to the identification of effective combination therapies and complementary approaches. Therefore, through this review paper, we aim to comprehensively review the existing management strategies for NP, specifically in the context of pharmacological, neuromodulation, and quality of life domains. The findings from this study will provide an elaborated understanding of the therapeutic modalities available and can help guide clinicians on the recent advances and efficacy of various modalities.
Methodology
A comprehensive literature search in the PubMed, Web of Science, Science Direct, and Cochrane databases utilizing the medical topic headings (MeSH) and relevant keywords such as ‘neuropathic pain’, 'management', ‘treatment’, ‘pharmacological’, ‘neuromodulation’, ‘quality of life', and a combination of all available related terms was performed on December 18, 2023. All relevant peer-reviewed articles involving human subjects and those available in the English language were included. Using the reference lists of the previously mentioned studies as a starting point, a manual search for publications was conducted through Google Scholar to avoid missing any potential studies. There were no limitations on date, publication type, or participant age.
Discussion
Many significant contributing processes such as abnormal ectopic activity in nociceptive neurons, peripheral and central sensitization, compromised inhibitory regulation, and pathological activation of microglia, are indicated by extensive evidence of NP research. A comprehensive history and physical examination are necessary for the clinical evaluation of NP in order to identify distinctive signs and symptoms. Clinical neurophysiological testing and further laboratory studies can frequently assist in determining the underlying cause and directing the choice of treatment (8). However, NP management continues to be an important concern, even with an increasing number of pharmaceuticals accessible. In accordance with systematic reviews and meta-analyses, the majority of drugs have dose-limiting adverse effects, and only a small percentage of NP patients respond adequately to pharmacological treatment. Recently, novel pharmaceutical interventions for NP have been investigated, involving the use of drugs that target novel pain receptors (9).
We briefly describe below various pharmacological and neuromodulation strategies employed in clinical practice and additionally assess the quality of life of NP patients.
Pharmacological therapeutic strategies
The pharmacological interventions for NP patients can be categorized into first, second, third- and fourth-line therapies and are briefly described below.
First-line therapy
Antidepressants
Among the earliest medications used to treat NP, antidepressants have been the focus of multiple randomized controlled trials. Considering some patients with chronic pain are also depressed, and since these medications reduce both pain and depression, they were first used to treat NP. Nevertheless, since the 1960s, tricyclic antidepressants (TCAs) have been shown to have an independent analgesic effect. Since then, people with chronic pain, both depressed and non-depressed, have reported pain relief. In certain patients, the alleviation appears to happen at a lower dosage than the antidepressant effect and can happen faster than usual (10). An early theory regarding the mechanism of antidepressant analgesia postulated that these medications could enhance the activity of the descending inhibitory pathways that run from the brain stem to the spinal cord's dorsal horn. This was achieved, in part, by preventing the reuptake of serotonin and noradrenaline that descender fibers release into the spinal synapses between spinothalamic neurons. Alternatively, they can cause interneurons to fire, which releases inhibitory chemicals like GABA or endogenous opioids (11).
In order to alleviate NP, antidepressant medications must be used over an extended period of time. This implies the involvement of secondary downstream mechanisms in addition to long-term molecular and neural plasticity. In the context of NP, noradrenaline plays a significant role in the antidepressant drug's activity. The recruitment of noradrenergic descending routes and peripheral noradrenaline recruitment from sympathetic fibers sprouting into dorsal root ganglia have been suggested by mechanistic concepts. The significance of both α2 and β2 adrenoceptors has been documented. The opioid system, specifically the mu and/or delta opioid receptors, is necessary for the therapeutic action of these monoamine reuptake inhibitors, which may also function indirectly as anti-proinflammatory cytokine medications (12).
Recent research has suggested that antidepressants may function through a peripheral mechanism. It seems that TCAs, specifically imipramine, desipramine, and amitriptyline and its metabolite, nortriptyline, are the most efficacious antidepressants for NP. TCAs are comparatively dirty drugs with pleiotropic actions that impact several targets. They are effective in part because of this lack of selectivity. Amitriptyline, for example, has been demonstrated to have a local anaesthetic effect through the blockage of voltage-gated sodium channels. As the immune system plays a major role in NP, antidepressants may have additional modes of action through immune system modulation. Moreover, by inhibiting NMDA receptors in the spinal cord, TCAs may directly impede central sensitization. TCAs have demonstrated effectiveness in treating a number of NP disorders, such as post-herpetic neuralgia and painful polyneuropathy, among others (13).
Sindrup et al. stated that one in two to three individuals with peripheral NP will be relieved by TCA, while a quarter of patients by serotonin noradrenaline reuptake inhibitors, and one in seven patients by selective serotonin reuptake inhibitors. Therefore, according to efficacy metrics like numbers needed to treat, TCAs typically outperform anticonvulsant gabapentin and treatment alternatives like oxycodone and tramadol; on the other hand, venlafaxine, a serotonin noradrenaline reuptake inhibitor, seems to be equally effective with these drugs, and selective serotonin reuptake inhibitors appear to be less effective (14). Similarly, the results of a meta-analysis demonstrated that neuropathic spinal cord injury pain can be effectively reduced by antidepressants. However, authors further commented that due to the small number of research studies available, this should be considered cautiously, and long-term treatment alternatives may need to be further evaluated (15). Urtis et al. described that antidepressant use provides individuals with chronic pain with alternative and complementary therapeutic alternatives (16).
However, results of a recent meta-analysis concluded that presently, there is insufficient and unreliable data regarding the effectiveness of antidepressants in the long run, nor is there any solid proof for their safety when used for chronic pain (17). Moreover, Moore et al. narrated that antidepressant medications can be used to treat NP at dosages lower than those that cause the medications to become antidepressants. For many years, the first-line treatment for NP has been amitriptyline. Although it is discouraging that there is no objective proof of a therapeutic effect, decades of successful therapy for many NP patients must be taken into consideration. While a small percentage of patients will get meaningful pain alleviation, amitriptyline should still be used in the treatment of NP (18).
Several adverse drug reactions that limit the use of TCAs are also caused by their numerous activities. The side effects of anticholinergic medications, which include dry mouth, orthostatic hypotension, constipation, and urine retention, are especially concerning due to the possibility of cardiotoxicity, which limits the daily dosage to less than 100 mg. Serotonin and norepinephrine reuptake inhibitors, especially duloxetine, have been used to treat NP in order to address these issues. Serotonin and norepinephrine reuptake inhibitors have demonstrated effectiveness in treating a number of neuropathic diseases, such as low back pain, painful diabetic neuropathy, post-herpetic neuralgia, and painful polyneuropathy (19). More research is needed, however, to demonstrate that the advantages of antidepressants in the management of NP outweigh their adverse effects and also assess their efficacy in the long term.
Gabapentin and pregabalin
For NP resulting from SCI, pregabalin and gabapentin are advised as the first line of treatment. It has been demonstrated that both medications are useful in treating NP caused by diabetic peripheral neuropathy and postherpetic neuralgia. The newest class of gabapentinoids, referred to as pregabalin, functions similarly to gabapentin. The U.S. Food and Drug Administration has licensed only one drug, pregabalin, for the treatment of NP in patients with SCI. Many individuals with SCI continue to experience pain even after receiving the recommended treatments. A number of recommendations regarding the safety and effectiveness of gabapentin and pregabalin in the treatment of NP following SCI have been published in recent years (20).
Findings of a study by Al-Ameri et al. concluded that pregabalin and gabapentin both efficiently and safely reduce the NP linked to the syndrome of failed back surgery (21). Similarly, findings of a meta-analysis of recent times concluded that pregabalin was the most effective drug for treating NP in individuals with SCI, whereas gabapentin fared better in terms of drug therapy-related safety (22). Results of another meta-analysis also concluded that pain and other secondary disorders post SCI seem to respond well to gabapentin and pregabalin treatment; however, patients must have close monitoring for side effects (23). Moreover, Bashford et al. described that the anti-epileptic drugs gabapentin and pregabalin are advised as first-line treatments for NP in accordance with evidence-based guidelines. They are taken orally and function by binding to the voltage-gated calcium channel's α2δ subunit to reduce pain. Comparing both medications against a placebo, they exhibit moderate but established efficacy. For pregabalin, the number needed to treat is 7.7, and for gabapentin, it is 6.3. Both gabapentin and pregabalin have comparable adverse effect profiles and are generally well tolerated. The most frequent results are somnolence and dizziness, which affect about 25% of patients (24).
Both pregabalin and gabapentin are recognized as first- and second-generation α2δ ligands, respectively, and can be used as supplementary therapies for pain management. They have been effectively utilized to treat NP syndromes despite not binding to GABA receptors. Although their exact mode of action is still unknown, research has yielded favourable results. Despite their similarities, they have been combined in clinical and research settings, and without fretting about pharmacokinetic interactions that are clinically important, they have been shown to have a synergistic impact in pain management. When compared to using a single medication, this combined method can be used to improve therapeutic response, lower the dose of an individual agent, and minimize its adverse effects. However, authors further suggested that before gabapentin and pregabalin combination therapy is suggested as the first line of treatment for patients with refractory pain and low levels of tolerance for an individual agent, pharmacokinetics, drug interactions, and adverse reactions to combinations must be taken into account (25).
Second-line therapy
Opioids
Tramadol and opioid analgesics are usually recommended as second-line treatments, although they may be used as first-line in certain clinical situations (26). Some of the research studies discuss opioids as third-line therapeutic agents; however, for this study, we have considered them as second-line therapy. As a second-line treatment for NP, tramadol has been demonstrated in randomized controlled trials to be beneficial for both mixed NP syndromes and diabetic neuropathy. It is a weak serotonin and norepinephrine reuptake inhibitor and a mild agonist of the µ-opioid receptor. When compared to other weak analgesics, tramadol may result in less nausea and constipation. Tramadol can lower seizure thresholds and raise the risk of serotonin syndrome when taken with other serotonergic medications, in addition to the typical opioid adverse effects. It emerged in certain research studies that opioids were superior to placebos in terms of pain relief. However, opioids are regarded as second-line agents for NP due to their possible side effects, medical complications including endocrine dysfunction, sleep apnea, opioid-induced hyperalgesia, risks such as overdose, diversion, addiction, withdrawal, and the need for more specialized follow-up and monitoring (6).
Because of its opioidergic and monoaminergic properties, tramadol has long been utilized as a well-tolerated substitute for other medications in the treatment of moderate pain. Over the past few years, however, significant evidence has been accumulated to suggest alternative plausible mechanisms and applications of tramadol in pain management. Tramadol modulates a number of mediators involved in pain signalling, including adenosine receptors, glutamate receptors, α2-adrenoceptors, voltage-gated sodium ion channels, transient receptor potential V1 channels, substance P, calcitonin gene-related peptide, prostaglandin E2, and proinflammatory cytokines. In both peripheral and central locations, tramadol also alters the crosstalk between neuronal and non-neuronal cells. Tramadol has the potential to regulate both central and peripheral neuronal hyperexcitability through these chemical mechanisms (27). Almost 16 randomized studies for chronic non-cancer pain were included in a meta-analysis of opioids for chronic pain. While some of these trials concentrated on postamputation pain, sciatica, and SCI pain, the majority of these studies investigated painful diabetic neuropathy and postherpetic neuralgia. With a moderate effect size (0.56) for pain, the authors observed that opioids were more effective than a placebo,13 of these RCTs showed a minor effect size (0.24) favouring opioids for function. When treating painful polyneuropathy and postherpetic neuralgia, the combined number needed to treat for opioids was 2.6 (28, 29).
Sommer et al. revealed in their findings that for 4–12 weeks, some opioids buprenorphine, morphine, oxycodone, tramadol, and tapentadol significantly reduce pain when compared to a placebo in cases of postherpetic neuralgia and peripheral neuropathies of various etiologies. However, the notion that these medications work well for treating other NP syndromes is not well supported by the data (30). While Li et al. reported that numerous meticulously designed clinical trials have demonstrated the analgesic effectiveness of opioids in the treatment of NP and malignant NP. Nevertheless, in order to confirm their distinct therapeutic effects and offer stronger evidence to direct clinical practice, more prospective randomized studies are particularly needed, given their wide range and formulation, distinct pharmacological properties, and distinct processes (31). The association between major side effects and dependency on opioids may limit their use in clinical practice; however, if their benefits outweigh these risks, this can only be answered through further research, specifically clinical trials.
Capsaicin and lidocaine
For patients with peripheral NP, lidocaine and capsaicin are suggested as second-line therapies. Lidocaine patches act by inhibiting voltage-gated sodium channels and temporarily reducing spontaneous ectopic nerve firing (4). When compared to systemically administered medications, topical therapies have the following advantages of site-specific drug delivery; avoidance of major drug interactions, infections, first-pass metabolism, and systemic side effects; and the requirement for a lower total systemic daily dose for patients to achieve pain relief. It has been demonstrated that a variety of topical medications are effective in treating people with chronic pain. Topical versions of capsaicin and lidocaine have been demonstrated to be effective in treating diabetic peripheral NP and postherpetic neuralgia in patients with NP. Lidocaine has also shown efficacy in treating patients with NP who are additionally suffering from complicated regional pain syndrome; however, results from certain clinical trials are not consistent with this (32).
Capsaicin, a naturally occurring spicy substance, has long been used topically. Once applied to the skin, capsaicin causes the local pain nerves to become temporarily more sensitive before gradually becoming less sensitive over time. Pain nerve fibers that express the transient receptor potential vanilloid-1 are stimulated to cause this. The greater dose of 8% topical capsaicin offers some of the best data currently available in the treatment of post-herpetic neuralgia and other neuropathic diseases, but low-dose capsaicin has not shown strong efficacy (33). Results of a clinical trial in recent times showed that, as no withdrawals occurred due to severe drug-related treatment-emergent adverse events, the capsaicin 8% patch seems to be rather well tolerated (34).
Third line therapy
Cannabinoids
When other forms of treatment are ineffective for a patient's condition, cannabis-based medicinal extracts may be able to effectively relieve their pain in various kinds of chronic non-malignant NP patient groups (35). Strong evidence supports the therapeutic use of products derived from cannabis, and scientific research has confirmed the effectiveness of phyto-, synthetic-, and endocannabinoids in the management of pain. The objective of using medicinal cannabis products is to enhance patients' wellbeing in several ways, in addition to reducing the symptoms of chronic pain (36). The mechanisms of action of cannabinoids include blocking the release of neurotransmitters from presynaptic nerve endings, adjusting postsynaptic neuronal excitation, triggering descending inhibitory pain pathways, lowering oxidative stress and inflammation in the brain, and repairing autophagy abnormalities (37).
There have been comprehensive systematic reviews addressing the application of cannabis in the management of chronic pain. Cannabinoids showed a moderate degree of support when used to treat chronic non-cancer pain. Long-term cannabis uses safety and its potential side effects are still unknown, though. According to some recent research, the management of NP may benefit from the use of novel cannabis derivatives and delivery systems that reduce the potential side effects of systemic dosing. By achieving a better pharmacokinetic/pharmacodynamic profile, these novel cannabis delivery methods may enable optimal adherence to therapy, hence increasing therapeutic options and improving clinical results. Cannabinoid derivatives have been suggested as having potential benefits in the treatment of neuroinflammation and NP. However, in patients with chronic NP, the possible risks of cannabis-based medication could outweigh its benefits (38).
BTX-A
As a powerful neurotoxin that inhibits synaptic exocytosis and therefore neuronal transmission, BTX-A is also included as a third-line treatment for spasticity. It has been demonstrated that subcutaneous injection of BTX-A is beneficial for patients experiencing allodynia and focal peripheral NP. In accordance with certain guidelines, BTX-A should only be used as a last resort in situations of peripheral NP that are not responding to other treatments (19).
Fourth-line therapy
Fourth-line therapies for NP can include methadone, various anticonvulsants, selective serotonin reuptake inhibitors and other miscellaneous agents. When it comes to managing NP, selective serotonin reuptake inhibitors seem to have a minimal analgesic impact. Independent of their antidepressant effects, citalopram, paroxetine, and escitalopram have been proven to be effective in the treatment of painful diabetic neuropathy and painful polyneuropathy, while fluoxetine has not. Selective serotonin reuptake inhibitors that are mainly prescribed for depression may hinder TCA metabolism and elevate the risk of serotonin syndrome (39).
Neuromodulation
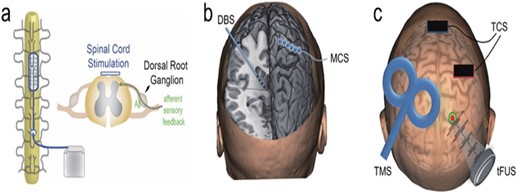
With neuromodulation, pre-identified neural targets in the central nervous system receive controlled physical energy, which is becoming one of the clinical methods for treating persistent NP. Neuroscience research and therapeutic practices are paying more and more attention to it since it has superior targeting, is drug-free, and does not cause any addiction. By introducing physical energy into the body, the rapidly developing discipline of neuromodulation provides modulative effects on the central or peripheral neural systems. It is applicable to the study of the brain and the treatment of brain diseases. Depending on the stimulation levels and applications, these technologies can be utilized to excite, inhibit, or disturb brain network dynamics in a regulated manner (40). (Figure 1) gives a pictorial representation of a few of the neuromodulation strategies. While some of the neuromodulation techniques are briefly described below. These techniques can be broadly classified as invasive and non-invasive neuromodulation procedures.
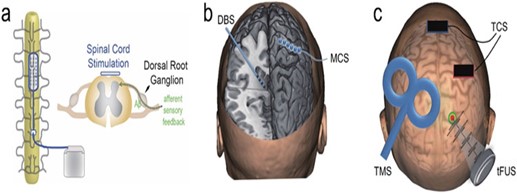
Figure 1: Technologies for physical neuromodulation. A diagrammatic representation of a spinal cord stimulation (SCS). b) A demonstration of an invasive approach, such as motor cortical stimulation (MCS) and deep brain stimulation (DBS). c) An illustration of transcranial magnetic stimulation (TMS), transcranial current stimulation (TCS), and transcranial focused ultrasonic stimulation(tFUS) (40)
A) Invasive neuromodulation
Spinal cord stimulation (SCS)
For more than 40 years, localized persistent medically refractory NP affecting the limb or limbs and trunk has been treated with spinal cord stimulation (SCS). Peripheral neuropathy, failed back surgery syndrome (FBSS), and complex regional pain syndrome (CRPS) continue to be the most common symptoms. The patient's perception of stimulation-induced paresthesias undermines the validity of the tonic SCS efficacy by preventing blinded evaluation and amplifying the placebo effect. The long-term improvement is lower for patients, while the short-term improvement is greater for approximately 50% of patients. Studies on CRPS have shown that SCS with physiotherapy is more effective than physiotherapy alone. Clinical investigations in FBSS have demonstrated that SCS is superior to traditional medical treatment and reoperation, respectively (41). Recently, new stimulation waveforms have been proposed to prevent paresthesias and/or improve pain relief: burst, high frequency (10 KHz) stimulation, and close-loop SCS stimulation. According to clinical experiments conducted in FBSS, these new SCS modalities were possibly even slightly more effective than traditional tonic SCS, if not more so. Few clinical investigations, as opposed to CMM, supported the effectiveness of SCS in treating excruciating diabetic neuropathy. Although common complications include hardware malfunction or migration, surface infections are quite hazardous. Since patients with chronic pain who undergo this operation are typically resistant to all other therapies, the risk-benefit ratio is in favor of SCS (41).
Deep brain stimulation (DPS)
By implanting electrodes into the deep brain, DBS, which was originally presented to the central nervous system by Hassler et al. in the middle of the previous century, offers excellent specificity and therapeutic efficiency. Numerous studies have shown that chronic DBS can cause analgesia in both human and animal subjects when it targets the lateral and medial nuclei of the sensory thalamus, the internal capsule, the periaqueductal/periventricular gray matter for the sensory aspect of pain, and the anterior cingulate cortex for the perception of pain. The most recent DBS system consists of an implantable pulse generator (IPG), connection cables connecting the DBS leads to the IPG, a patient programmer, and a small DBS lead that is inserted either bilaterally or unilaterally directed into the brain region (40).
Many prospective case series have been documented for the treatment of chronic NP unresponsive to medicinal therapy. However, few published findings from patients treated with current standards of neuroimaging and stimulator technology during the previous ten years have been published. For certain patients, particularly those with pain following amputation, brachial plexus damage, stroke, and cephalalgias, including anaesthesia dolorosa, several facilities continue to use DBS for chronic pain with effectiveness. Additional achievements include pain following a spinal injury and multiple sclerosis. Somatotopic coverage is crucial during an awake operation. In cases of whole or hemi body pain or following the failure of DBS on other targets, cingulate DBS under general anaesthesia may be considered (42).
B) Non-invasive
1. Transcranial current stimulation
The use of electrical currents to alter brain function is referred to as transcranial direct current stimulation (tDCS), and it is quite an old technique dating back to over 200 years. However, it was reintroduced approximately 25 years ago. With tDCS, a safe non-invasive procedure, scalp electrodes are used to provide a low amplitude electrical current to the cortex. An apparatus using tDCS requires two fundamental parts: an electrode set and a power supply. Nine volts of direct current are used as the power source, and two surface-conductive electrodes carry this voltage. The electrodes are coated with sponges that have been soaked in gel or saline to reduce impedance. The electrode sizes employed are 25–35 cm2, which are appropriate for a steady focality and current density. An appropriate current density of 0.029 to 0.08 mA/cm2 is supplied (43). Research indicates that tDCS has the ability to significantly lower NP, at least initially. The results underscore the necessity for larger-scale clinical trials aimed at determining: the optimal dosage of triamcinol tDCS including number and frequency of treatment sessions to maximize benefits; the duration of benefit maintenance and the number of patients affected; the necessity of booster tDCS sessions to support long-term benefit maintenance; and the most effective way to combine tDCS with other treatments to maximize overall treatment efficacy for pain reduction and quality of life enhancement in people with NP (43).
2.Transcranial magnetic stimulation (TMS)
For pain syndromes including NP, migraine, and fibromyalgia, non-invasive brain stimulation TMS is a well-tolerated and sensible adjunctive therapeutic option. Although research on methods to increase its efficacy is still proceeding (44). Given the limited effectiveness of pharmacological therapies and the prevalence of their side effects, repeated TMS is seen as a promising non-invasive approach for the management of chronic NP. Chronic NP can be significantly relieved by repetitive TMS (45). Using a coil of wire, TMS produces rapidly varying magnetic fields that induce electromagnetic induction and eddy currents in the brain, eliciting synchronized brain activities at the cortical brain areas with a resolution of a few centimeters at a time, in accordance with Faraday's law. The focality and penetrating depth of TMS still need to be enhanced in order to have more targeted effects in managing brain diseases, even though it has been shown to be useful in treating disorders such as pain, depression, stroke, and Parkinson's disease. Motor cortex stimulation is primarily used to relieve pain because TMS cannot focally excite deep brain regions. Because of plasticity, pain reduction after daily stimulation lasts for days to weeks (40).
Quality of life
The impact of NP on mood and quality of life can be profound. The Beck Depression Inventory, the PainDETECT Questionnaire, the Hospital Anxiety and Depression Scale, the Depression, Anxiety and Stress Test, and the Profile of Mood States can all be used to gauge this effect. These questionnaires can be filled out during the initial consultation to determine if there is an impact. If so, the allied health professional team can then conduct a more thorough assessment. When it comes to measuring the extent of catastrophizing, its effects on mood and quality of life, coping mechanisms, and kinesophobia, psychologists are vital. A wide range of validated instruments are typically utilized for this, such as the Pain Coping Inventory, the Tampa Scale of Kinesiophobia, and the Pain Catastrophizing Scale. Quality of life associated with a specific condition is measured using the Brief Pain Inventory, whereas general health-related quality of life is measured with the SF-36 or EuroQol-5D (46).
O’ Connor described that among patients with NP, health-related quality of life (HR-QOL) is substantially decreased. Patients report diminished mobility and job capacity as well as pain-related interference in several HR-QOL and functional domains. In addition, it has been demonstrated that NP has negative social repercussions for the spouses of NP patients. Multiple drugs have been demonstrated to improve different measures of HR-QOL in randomized controlled trials. Reductions in pain seem to be closely associated with changes in HR-QOL, but not with the emergence of adverse consequences. In spite of using NP-prescribed medicine, many patients in cross-sectional studies report having moderate to severe pain and noticeably worse HR-QOL. It seems that the standard of NP care is low, with few patients obtaining suggested therapy (47).
Moreover, Lu et al. reported that depression and a poor quality of life are prevalent in NP patients. The relationship between pain, despair, and quality of life is not well understood, though. Patients with NP experience some unfavorable effects on their quality of life from both depression and pain intensity. In individuals with NP, depression acts as a mediator between pain intensity and quality of life. Clinicians treating NP patients should regularly monitor depression in their patients and endeavor to reduce it (48). Studies from the literature demonstrate that NP patients have impaired quality of life. Our review comprehensively delves into the therapeutic modalities employed for the management of NP and provides evidence from recent times; however, it was beyond the scope of this study to describe each neuromodulation strategy more elaborately and may have overlooked some strategies that we aim to discuss in our successive studies in the future.
Conclusion
NP can be highly incapacitating, challenging to identify, and only partially alleviated by almost all forms of therapy. Additionally, it significantly impairs the quality of life of patients and their families, therefore a systematic, interdisciplinary strategy is required to help patients achieve a satisfactory quality of life and reduce pain. Recent guidelines suggest certain pharmacological and neuromodulation interventions which show promising outcomes also however they are associated with certain adverse effects also for which further research focusing on increasing the benefits and efficacy and reducing the adverse profiles is need of time.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.