Volume 3, Issue 12
December 2023
Local Delivery and Controlled Release Drug Systems in Periodontal Diseases
Maha Munshi, Razan Alhumaid, Alwaleed Almotawa, Nora Khashab, Alaa Albasher, Muhab Alzain, Rawan AlAnazi, Abrar Aljafar, Muhammad Althowaini, Esraa Alzahrani, Ssamar Hamdi
DOI: http://dx.doi.org/10.52533/JOHS.2023.31203
Keywords: Local drug delivery system, periodontitis, tooth, infection, periodontal disease
Periodontal disease, a prevalent chronic inflammatory condition impacting the structures supporting teeth, remains a leading cause of adult tooth loss. This ailment involves inflammatory processes around teeth, resulting in the degradation of supporting tissues, including bone and connective tissues. The formation of pockets between teeth and gums becomes a breeding ground for bacteria, exacerbating the infection. Treatment strategies encompass a blend of professional dental care, self-care routines, and, when necessary, surgical interventions. The primary objectives are infection control, inflammation reduction, and prevention of further damage to tooth-supporting structures. The emergence of microbial resistance and challenges like poor tissue penetration with conventional antibiotic use prompted the development of local delivery drug systems for periodontitis. Local drug delivery systems specifically target bacteria in the inflamed periodontal pocket, releasing active drugs in a controlled manner. This approach aims to manage periodontitis effectively while minimizing systemic dissemination of drugs. This review article aims to offer a comprehensive overview, exploring the current state, clinical applications, challenges, and potential advancements in local drug delivery and controlled release systems for treating periodontal diseases.
Introduction
Periodontal disease is a chronic inflammatory condition that affects the tissues surrounding and supporting the teeth. It is one of the most common oral health issues and is a major cause of tooth loss in adults (1). Periodontal disease is typically caused by bacterial infection and inflammation caused by the immune system in response to it. There are two main stages of periodontal disease: Gingivitis and periodontitis. Gingivitis is commonly characterized by mild inflammation of the gingiva and is usually reversible. Symptoms include redness, swelling, and bleeding of the gums during brushing or flossing, and can be reversed with good oral hygiene practices. However, if the inflammation progresses due to untreated gingivitis, it can change into periodontitis. Periodontitis involves inflammation around the tooth that leads to the destruction of the supporting structures, including bone and connective tissue. Pockets may form between the teeth and gums, providing a space for bacteria to thrive and exacerbate the infection (2).
Multiple causes and associated risk factors have been identified for periodontal diseases. The primary cause of periodontal disease is the accumulation of bacterial plaque on teeth and gums. Plaque is a sticky film of bacteria that forms on teeth and can harden into calculus if not removed. Periodontal disease can also be attributed to other risk factors such as tobacco use, genetic predisposition, co-morbidities, and hormonal changes (3). Based on pathophysiology, periodontitis can be further classified into periodontitis, periodontitis associated with systemic disease, and necrotizing periodontitis (4).
Periodontitis has been identified as the sixth most prevalent disease in the world (5). In 2019, 1.1 billion cases of periodontitis were reported, and it is assumed that the real estimate, including the unreported cases, is far larger (6). The global prevalence of severe periodontitis was estimated to be 11.2% and had a gradual ascending trend when charted with age, showing peak incidence at 38 years of age (5). Considerable variances were observed in the prevalence of periodontitis among different regions of the world, with a higher prevalence in low-middle-income countries (7). Between 1990 and 2017, the prevalence of periodontitis in China, Japan, and India was observed to be increasing, however, it decreased in South Korea and Thailand. The highest rates of periodontitis were found to be in India, with an overall prevalence reported to be approximately 51% (8, 9).
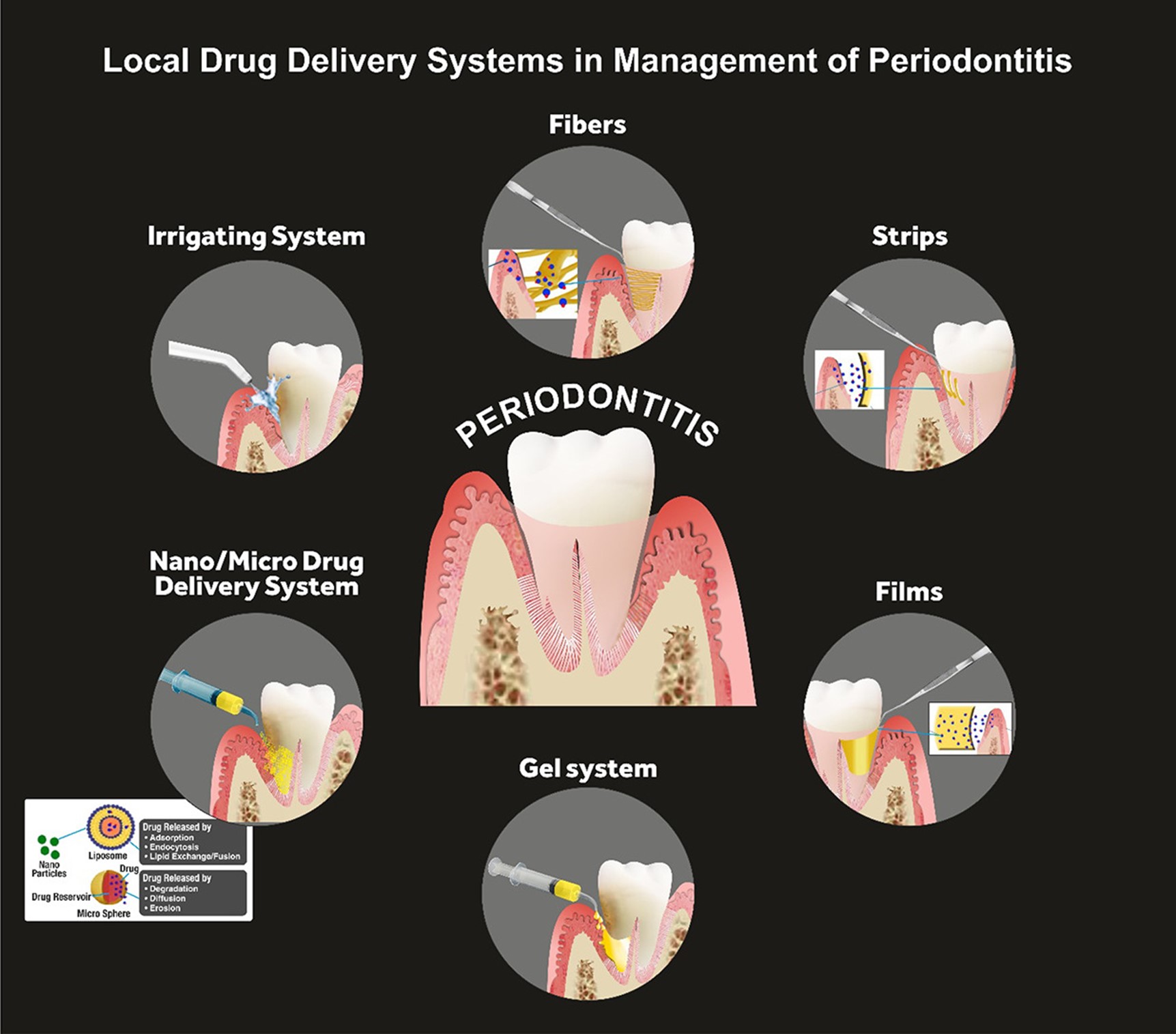
The treatment of periodontitis involves a combination of professional dental care, self-care practices, and, in some cases, surgical interventions. The goals of treatment are to control the infection, reduce inflammation, and prevent further damage to the supporting structures of the teeth (10). The fundamental non-invasive conservative treatment strategy is scaling and root planing, which involves the removal of the calculus and smoothening of the root surfaces to prevent bacterial accumulation. In addition to that, bacterial mouthwashes and better brushing habits are also introduced, along with lifestyle changes related to smoking and oral hygiene maintenance (11). Other treatment regimens corresponding to progressed periodontitis are laser therapy, pocket reduction surgery, bone and tissue grafting, guided tissue regeneration, and maintenance therapy. These are surgical procedures that are preferred when significant bone resorption along with severe attachment loss is observed. In some cases, antibiotics may also be prescribed to control bacterial infections (12). Systemic drug administration for periodontitis has been proven to be effective for the past half-century, however, multiple problems with systemic drug intake have been identified. Issues such as poor biodistribution lead to the administration of high doses of medicines so that effective concentration for treatment can be achieved, which eventually leads to toxicity, gastrointestinal problems, and drug resistance (13). To address these disadvantages, LDDS is now more commonly preferred over systemic drug administration. Directly addressing the shortcomings of systemic drug delivery, LDDS is placed inside the periodontal pockets (Figure 1), providing efficacious active drug concentration with a minimum and controlled dosage. Further advantages include fewer side effects, such as gastrointestinal imbalance. This is particularly important in the treatment of chronic conditions like periodontal diseases, where long-term drug therapy may be necessary (14).
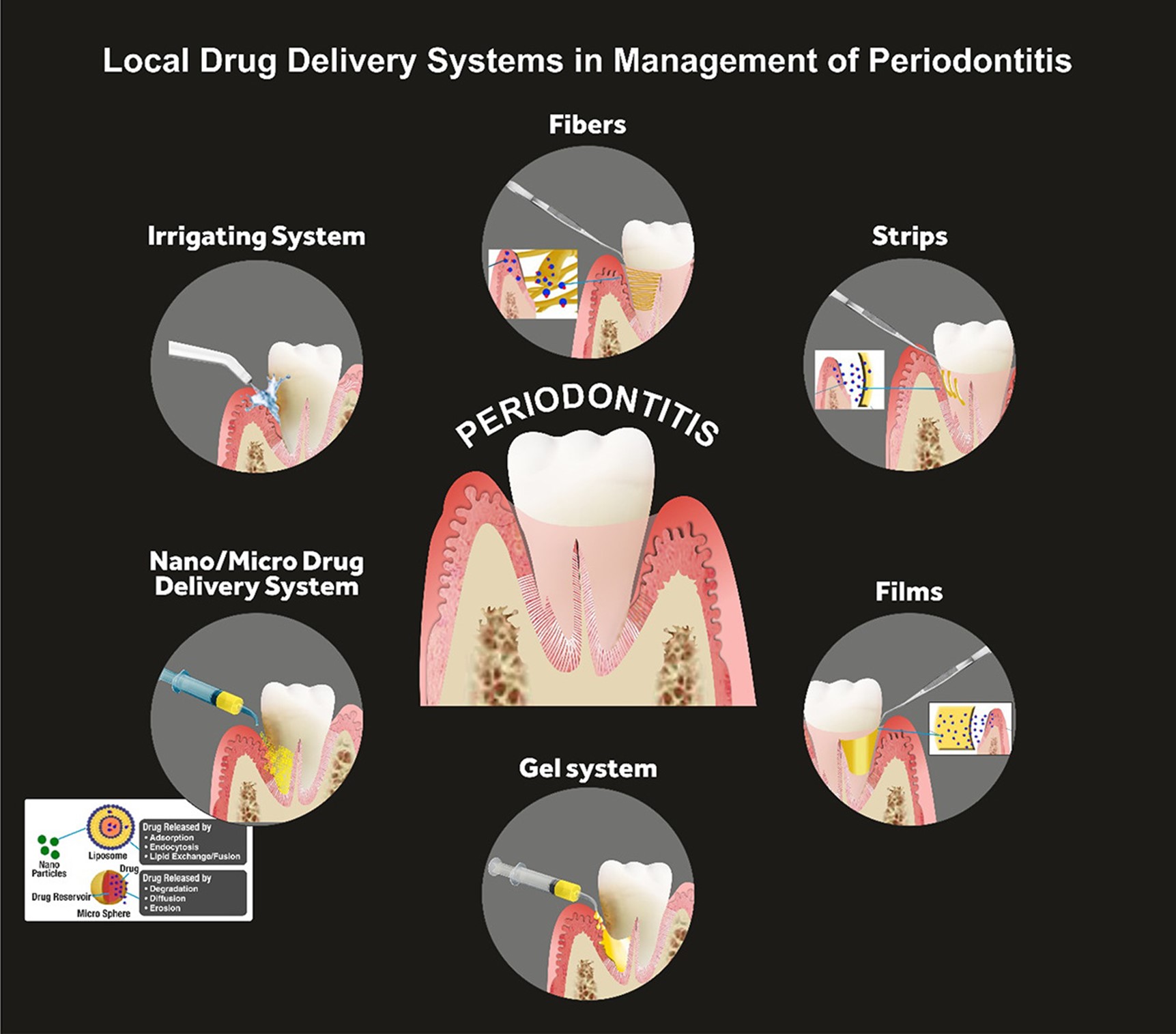
Figure 1: Local Drug Delivery Systems (LDDS) in management of periodontitis (21)
The study aims to comprehensively review and evaluate the efficacy of local delivery and controlled release drug systems in the context of periodontal diseases. The rationale for this investigation is grounded in the need for advanced therapeutic strategies that can enhance treatment outcomes for periodontal diseases. The investigation seeks to provide a comprehensive synthesis of current knowledge, shedding light on the clinical applications, challenges, and potential advancements in local drug delivery and controlled release systems for periodontal diseases. Ultimately, the study endeavors to contribute valuable insights that can inform future research directions and guide the development of innovative therapeutic approaches for the management of periodontal diseases.
Methods
Initiated on November 12th, 2023, the research project commenced following an exhaustive examination of existing literature. Multiple databases, including PubMed, Web of Science, and Cochrane, were employed for an extensive literature review. Various medical terms were utilized in diverse combinations during the search process. Additionally, manual searches on Google Scholar were conducted to identify pertinent research terms. The literature review primarily focused on key areas such as the prevalence of periodontitis, available treatment options, and the utilization of local drug delivery systems with controlled drug release. The search strategy also incorporated keywords about the types and efficacy of local drug delivery systems. It's crucial to highlight that article selection criteria were established based on multiple factors to ensure a thorough and robust review process.
Discussion
Local drug delivery systems enable the targeted administration of drugs to the periodontal pockets, where infection and inflammation are concentrated. This targeted approach enhances the concentration of the therapeutic agent at the site of action, increasing its efficacy. Controlled release systems are also advantageous in managing chronic conditions, as they help maintain therapeutic levels of the drug at the target site, reducing the frequency of administration and improving patient compliance (15).
Types of LDDS
Fibers
Fibers are hollow drug delivery appliances that are usually placed with an adhesive inside a periodontal pocket for the treatment of periodontitis (16). They can be made up of either natural polymers, such as gelatin, or synthetic polyurethane and polypropylene. These fibers are loaded with antibiotics like tetracyclines and are biodegradable (17). Multiple studies and trials have endorsed the efficacy of these drug-loaded fibers for the management of periodontitis. Remarkably reduced pocket depths were observed in a study conducted in 2009 where biodegradable collagen fibers loaded with tetracycline were used as an adjunct with scaling and root planing (18). This outcome was further supported by another randomized clinical trial where tetracycline-loaded fibers were used as an adjunct in severe periodontitis (19). Another study stated that the degradation of antibiotic fibers was found to be correlated with controlled drug release, acting as a potential clinical indicator for the efficacy of the drug release system (20).
Strips and films
Strips and films consist of thin matrix bands that are specifically designed for uniform drug dissolution through the polymer, making them suitable for large periodontal pockets (21). Recently, bioabsorbable materials like poly-hydroxybutyric acid and gelatin have been introduced, which have shown promising results in the literature. Long-term maintenance of drug concentration and resulting improvements in gingival health have been some characteristics of strips and films quoted in the evidence (22). Various studies endorsed a reduction in probing depths after using chlorhexidine or tetracycline-loaded strips and films as an adjunct with scaling and root planing (23, 24). Strips and films use similar materials and mechanisms as fibers however differ in size and drug release rate. While fibers are suitable for inaccessible regions, SFs, being wider, are more appropriate for larger pocket areas (25).
Microparticles
Microparticles, defined as solid spherical polymer structures, serve as a versatile platform for drug delivery. Loaded with drugs dispersed uniformly within the polymer matrix, microparticles offer easy administration and prolonged drug release and can be inserted as chips, dental pastes/gels, and direct injection into the periodontal pocket (21). Antibiotics such as minocycline and metronidazole-loaded microparticles have been deemed efficient for the management of periodontitis (26, 27). Clinical investigations have explored the application of microparticles in periodontitis treatment, with solid lipid microparticles (SLMs) gaining attention. Gad et al. (28) developed SLMs encapsulating doxycycline hydrochloride and metronidazole, demonstrating high biocompatibility, drug stability, and a significant decrease in anaerobic bacteria with the adjunctive use of SLM gel to conventional scaling and root planing in patients with chronic periodontal disease. The study showcases the potential of SLMs as an effective local drug-delivery system in periodontal therapy.
Nanosystems
Due to their minute sizes, nanosystems are easier to administer in areas that are usually inaccessible in periodontal treatment, such as the periodontal pocket below the gingival line (21). Micelles, metallic and polymeric nanoparticles, liposomes, and nanofibers constitute nanosystems that also exhibit antibacterial properties. Polymeric nanoparticles, particularly those with poly (lactic-co-glycolic acid) (PLGA) and chitosan-based nanoparticles loaded with doxycycline showcase good cell compatibility, antibacterial efficacy against, periodontitis and downregulation of inflammatory factors (29). Another study highlighted the efficacy of doxycycline-loaded nanosystems used as an adjunct with scaling and root planing (30).
Gels
Gels are usually administered through needle syringes directly into the periodontal pocket and are typically made up of polymers like Carbopol, carboxy methyl cellulose, and chitosan (31, 32). Gels can also transform from liquid to semisolid state in response to temperature stimuli and are recorded to be efficient in periodontal patients for delivering doxycycline and metronidazole (28). Moreover, gels loaded with anti-inflammatory drugs, osteogenesis drugs, and natural agents have shown positive results in terms of periodontal health and sustained drug release. These formulations represent promising alternatives to antibiotic-loaded gels, addressing the challenge of bacterial resistance. A study explored the efficacy of a thermosensitive gel loaded with aspirin and erythropoietin which demonstrated controlled drug release and biocompatibility (33). The versatility and efficacy of gels, either alone or in combination with other locally delivered drug systems, make them a preferred therapeutic option in periodontal treatment, offering patient comfort and acceptability.
Membranes
In cases of advanced periodontitis, bone resorption is evident and is a crucial defect leading to tooth mobility. Promotion of bone regeneration through the insertion of relevant growth factors has been an area of interest. Membranes act as barriers that facilitate wound healing and bone regrowth through the introduction of growth factors in the periodontal tissues (34). Membranes are currently made up of collagen or polyglycolide copolymers and are immune compatible, hemostatic, and stimulate the gingival fibroblast cells (35). Studies using bone regeneration membranes have highlighted their efficacy in inhibiting alveolar destruction and recovering bone defects, positioning them as a potential future therapeutic alternative for periodontitis (36). While membranes loaded with growth factors for bone regeneration have been explored, antibiotic-loaded membranes have also been investigated. Amoxicillin-loaded membranes have demonstrated controlled drug release, aiding in the management of periodontitis (37). Despite the promising results, the study underscores the need to explore formulations loaded with alternative therapeutic agents, considering the contemporary challenges associated with antibiotic use in clinical settings.
Scaffolds
Scaffolds, which address bone defects like membranes, are proven to be more resilient to external mechanical forces. These scaffolds are strategically placed in affected areas to preserve space for the subsequent regeneration of periodontal tissues (38). Antibiotic-loaded scaffolds have proven their efficacy in multiple studies and are currently being explored for potential future applications (39). In addition to that, stem cells can also be incorporated into the scaffolds to promote periodontal regeneration. A piece of evidence explored the incorporation of mesenchymal stem cells for periodontal regeneration through scaffolds, showing improvements in clinical attachment level, pocket depth, and linear bone growth (40).
Challenges
Local drug delivery systems (LDDSs) exhibit notable advantages, yet they are not without challenges. The management of certain LDDSs can be challenging, and achieving sufficient drug concentrations poses difficulties for some systems. Controlled drug release is a fundamental requirement, ensuring that the drug is dispensed in a regulated manner. Maintaining stable drug concentrations over an extended period is essential for sustained therapeutic effects (24). Early examples of these systems were non-biodegradable, necessitating a second intervention for removal, causing discomfort. Biodegradability is crucial, allowing for the system to naturally break down over time. Biocompatibility ensures that the LDDS is well-tolerated by the body, minimizing adverse reactions. Since LDDSs are relatively new, ongoing research is crucial to determine the most effective type of LDDS (20). Furthermore, the implementation of LDDSs can be hindered by their association with elevated costs, presenting a potential obstacle to widespread clinical application (21). Despite the challenges, ongoing research and advancements are aimed at overcoming these limitations. The evolving landscape of LDDSs holds promise for improved biodegradability, enhanced biocompatibility, simplified administration, and more controlled and sustained drug release. Addressing these challenges will contribute to the development of more effective and patient-friendly LDDSs, fostering their integration into mainstream clinical practice. The exploration of novel materials, formulations, and delivery mechanisms is essential to refine the design and functionality of LDDSs, ultimately maximizing their therapeutic impact while minimizing drawbacks.
Conclusion
LDDS addresses the issues posed by systemic drug delivery by placing drugs directly into periodontal pockets, ensuring effective concentrations with minimal dosage. LDDS offers advantages like targeted drug delivery, concentrating therapeutic agents at the inflammation site, enhancing efficacy, and minimizing side effects, which are crucial for chronic conditions like periodontal diseases. Controlled release systems improve patient compliance by maintaining therapeutic drug levels and reducing administration frequency in long-term therapies. The shift towards LDDS signifies a more effective and patient-friendly approach to periodontal treatment.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.