Volume 3, Issue 9
September 2023
Perioperative Haemodynamic Optimization Strategies in High-Risk Surgical Patients
Mazen Nassar, Emad Almohisin, Alaa Alnasser, Amani Alaida, Zakeyah Shahini, Burair Al Jassas, Ahmed Albahrani, Latifah Almutairi, Ahmed Alshaer, Fatimah Aljohani, Abdulrhman Mughallis
DOI: http://dx.doi.org/10.52533/JOHS.2023.30901
Keywords: surgical, high-risk, haemodynamic, strategies
Millions of surgical procedures are performed globally each year. High-risk surgical procedures have varying but generally, high mortality and morbidity rates, making them a global challenge. In order to lower the risk of postoperative morbidity and mortality, perioperative haemodynamic optimization strategies have been designed. They are also referred to as goal-directed therapy. These guidelines for managing high-risk surgical patients propose strict adherence to values of mean arterial pressure not to fall below 65 mmHg during surgery, as mean arterial pressure significantly influences organ perfusion. It is recommended to utilize dynamic indicators to guide fluid administration in a substantial portion of non-cardiac surgery cases. However, it is advised to implement a checklist of validity criteria prior to administering volume expansion, acknowledging the recognized limitations of these indices. To tailor fluid therapy appropriately for individual patients and prevent fluid overload, it is commonly recommended to employ minimally invasive or non-invasive monitoring of stroke volume/cardiac output, particularly for patients undergoing moderate- to high-risk surgeries. Furthermore, these strategies suggest combining fluid administration with vasoconstrictors to sustain optimal blood flow and maintain perfusion pressure above hazardous thresholds. Incorporating personalized hemodynamic optimization approaches to reduce postoperative complications, shorten hospital stays, and ultimately prevent mortality in high-risk surgical patients has become a crucial aspect of standard surgical care. This review aims to assess strategies for optimizing perioperative hemodynamics in high-risk surgical patients.
Introduction
Globally, the number of surgical procedures is increasing, and there is a clear need to enhance access to safe, timely, and cost-effective surgery. Despite the most recent technical advancements in anesthetic care, challenging surgeries are increasingly being offered to patients who are older and have significant comorbidities as overall life expectancy rises. As a result, mortality and morbidity after surgery inevitably rise. Postoperative morbidity and mortality vary widely among patients, but they are especially high in a vulnerable subset of high-risk patients. Modern anesthetic care faces an increasing challenge from the high-risk surgical patient (1). The mortality rate for high-risk patients is over 30%, and multiple organ failure that develops following the procedure is the primary cause of death (2).
The process of defining risk in surgical patients is intricate and mostly depends on the surgeon's and anesthesiologist's subjective assessment of the patient's general health status as well as the nature and urgency of the surgical treatment (3). It has been suggested to classify patients as high-risk surgical patients if they have an individual mortality risk of more than 5% or are undergoing a procedure with a mortality risk of more than 5%. Extremely high-risk surgical patients are categorized as those whose probability of perioperative mortality is estimated to be greater than 20% (4). In recent times, clinical research has supported the beneficial effects of perioperative hemodynamic optimization on surgical mortality and postoperative complication rates. In order to direct therapies to meet predetermined goals, this approach necessitates the use of advanced hemodynamic monitoring (5).
Low cardiorespiratory reserve and the failure to sustain adequate oxygen supply during surgical trauma to meet the increased metabolic demand are related to postoperative complications among high-risk individuals having major surgery. Hypoperfusion, multiple organ failure, and severe infections are a result of an imbalance in the ratio of oxygen delivery to oxygen consumption, which are significant contributors to postoperative mortality. To prevent hypovolemia or hypervolemia, tissue hypoperfusion, and surgical problems, perioperative hemodynamic optimization therapy tries to modify heart performance to match the increased demand during the perioperative period. This calls for sufficient hemodynamic monitoring to direct early patient care and enable faster detection of the need for fluid optimization, blood transfusion, and vasoactive medications (6). The purpose of this research is to review perioperative haemodynamic optimization strategies in high-risk surgical patients.
Methodology
This study is based on a comprehensive literature search conducted on August 11, 2023, in the PubMed, Web of Science, Science Direct and Cochrane databases, utilizing the medical topic headings (MeSH) and a combination of all available related terms, according to the database. To prevent missing any possible research, a manual search for publications was conducted through Google Scholar, using the reference lists of the previously listed papers as a starting point. We looked for valuable information in papers that discussed perioperative haemodynamic optimization strategies in high-risk surgical patients. There were no restrictions on date, language, participant age, or type of publication.
Discussion
Only 10-15% of surgical procedures are performed on high-risk patients, yet they are responsible for more than 80% of deaths. Patients who are admitted to the intensive care unit after receiving initial postoperative treatment in a normal ward have the highest mortality rates of almost 39% in this demographic (5). Perioperative haemodynamic strategies are also referred to as goal-directed therapy (GDT) and have been designed in an effort to reduce morbidity and mortality among patients. The decision about perioperative hemodynamic monitoring for GDT is based on both patient and surgery-related risk factors. Low- to moderate-risk patients can have their perioperative needs optimised using traditional monitoring and less invasive techniques. However, the most dependable procedures for major surgery and high-risk/unstable patients include thermodilution techniques and continuous cardiac output/oxygen transport monitoring. Maintaining cardiac function and organ perfusion while optimising the relationship between oxygen delivery and consumption is a key objective of perioperative hemodynamic treatment. GDT delivers optimal haemodynamic performance, enhances organ function, decreases the number of complications and time to intensive care unit and hospital discharge, and lowers the mortality rate in high-risk surgical patients, according to a variety of studies utilising various monitoring techniques and endpoints. GDT offers several advantages in major surgery. The goal-directed algorithms support the early detection of pathophysiological changes based on sufficient monitoring and have an impact on the perioperative hemodynamic therapy that can enhance the clinical outcome. Therefore, it is recommended that every patient receive a timely, sufficient, and customised GDT (7). The concept of perioperative optimization therapy is illustrated in Figure 1.
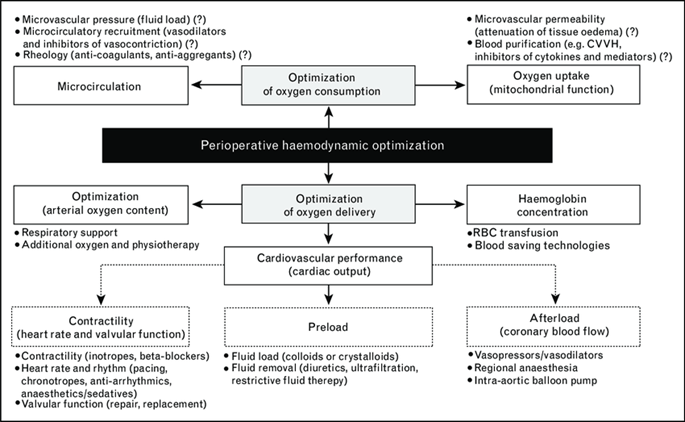
Figure 1: The concept of perioperative optimization therapy (7)
Strategies defined in literature.
Mean arterial Pressure (MAP)
During the administration of anesthesia, the continuous monitoring of blood pressure is a fundamental requirement due to its pivotal role in preventing postoperative complications arising from either low or high arterial pressure. The suggested range for maintaining mean arterial pressure (MAP) during the surgery is between 60 and 70 mmHg. In cases where patients have a history of chronic arterial hypertension and are undergoing elective non-cardiac procedures, it might be reasonable to consider aiming for MAP levels above 70 mmHg, contingent upon the specific clinical and surgical context. Additionally, an extensive randomized controlled trial conducted across multiple medical centres concentrated on high-risk surgical patients who were undergoing major abdominal surgery. The trial compared theeffectiveness of an individualized approach utilizing low-dose norepinephrine to maintain intraoperative systolic arterial pressure within 10% of the preoperative baseline, against a conventional standardized management strategy. The results demonstrated a statistically noteworthy reduction in postoperative organ dysfunction within the group that adopted the personalized approach (8, 9). Therefore, a personalized approach is highly recommended in this context.
In terms of organ perfusion, which is the primary determinant of survival, there is some evidence that increasing MAP with noradrenaline without clinical improvement of cardiac output is not beneficial. Deruddre et al. demonstrated that increasing MAP with noradrenaline from 65 to 75 mmHg was associated with a significant increase in cardiac and urine output as well as a considerable decrease in renal vascular resistance as measured using doppler ultrasonography. Despite the higher cardiac output, other research studies demonstrated that elevating MAP with noradrenaline above 65 mmHg on balance was not linked to better organ perfusion. In these studies, however, an increase in cardiac output following the first MAP escalation step from 65 to 75 mmHg was not clinically significant (>10%), and additional MAP increases in those studies did not appreciably increase the cardiac output (5, 10).
Fluid balance
The primary therapeutic measure essential for preserving or reinstating tissue perfusion amid surgical interventions involves the administration of intravenous fluids. Nonetheless, it is widely acknowledged that inadequate fluid replenishment can lead to adverse consequences, especially in patients who possess compromised health or are deemed high-risk individuals. Inadequate fluid administration may lead to diminished circulation and, in certain instances, insufficient perfusion in areas characterized by heightened vessel resistance, thereby affecting flow dynamics. Every organ may be included in these sites, which can vary from patient to patient. Because of the decreased local blood supply, cells may become hypoxic, which may lead to organ malfunction or failure. On the other side, if too much fluid is given, edema and venous congestion will develop. The effects of excessive fluid intake are likely just as harmful as those of hypovolemia, and numerous studies have demonstrated a direct link between a favorable fluid balance and complications following surgery (11, 12). Therefore, guidelines recommend that in normovolemic patients, the fluid strategy should aim for a near-zero balance before the start of surgery; nevertheless, to protect renal function, a minor positive fluid balance may be permitted. Only when fluids alone are insufficient to achieve optimal hemodynamics medications including inotropes, vasoconstrictors, and vasodilators shall be employed (13).
Mythen et al., however, narrated that recommendations are frequently unclear, with the use of phrases such as maintain adequate volemia or optimal volume that do not translate into quantitatively useful data being considered or utilized. Moreover, there are more concerning approaches that propose uniform administration of a predefined fluid volume to every patient, adjusting for variables like body weight and the duration of the surgical operation. This assumption assumes uniform requirements and tolerances across individuals. However, relying on this standardized approach is undoubtedly destined to result in suboptimal fluid management for the overwhelming majority of patients (14). Lamke et al. stated that the divergence from the acceptable volume needs will generally be tolerated by low-risk patients, but high-risk patients will be at risk for serious complications (15). It is therefore suggested that, including drug infusion, continuous fluid infusion be kept to less than 2 ml/kg/h (14).
The transition from invasive monitoring using pulmonary artery catheters to less or minimally invasive monitoring technologies is one of the important factors that has altered during the past ten years. The examination of fluid responsiveness using either dynamic indices or functional hemodynamic has replaced the use of static indices in the evaluation of intravascular fluid volume deficits, which has undergone a significant change. Finally, attention has been focused on crystalloid maintenance fluid techniques that are more limited. GDT is risk-free and more likely to match the fluid intake to what is truly required. This method entails evaluating fluid responsiveness and, if necessary, using inotropes. It can also be used in conjunction with a fluid restriction strategy for maintenance fluids. The use of fluids, vasopressors, and inotropes has improved as a result of these treatments being more frequently incorporated into protocols for perioperative hemodynamic optimisation in high-risk patients undergoing major surgery (16).
Cardiac output, oxygen delivery and tissue perfusion
Shoemaker presented the first observational evidence associating different cardiovascular measures with patient outcomes in those at high risk of mortality or experiencing complications following surgery, and he suggested that the emergence of tissue hypoxia was a possible explanation. Prior research had also revealed that survivors consistently had higher cardiac output, oxygen flow, and oxygen consumption than those who died once routine indicators like blood pressure and urine output were stabilized in all patients. The median levels obtained by surviving patients after being stabilized after surgery were the focus, not the cardiovascular characteristics of a normal person at rest. The most significant variables were the cardiac index (above 4.5 l/min per m2), oxygen intake (above 170 ml/min per m2), and oxygen delivery (above 600 ml/min per m2) (17) (18, 19).
The gold standard for evaluating central hemodynamic parameters and infusion response is cardiac output monitoring. There are numerous techniques for measuring cardiac output, each with a different level of invasiveness and a different continuous or intermittent study strategy. In the case of the most challenging individuals, transpulmonary thermodilution or pulmonary artery catheterization is used to identify the type of shock. Figure 2 displays the indications for the various cardiac output evaluation techniques used during the perioperative hemodynamic care of surgical patients. Invasive uncalibrated pulse wave analysis or esophageal doppler can be utilized to direct cardiac output optimization in high-risk non-cardiac surgery patients without noticeably altered vascular tone. Transesophageal echocardiography is recommended for individuals undergoing open-heart and thoracic aortic surgery. The use of transesophageal echocardiography during coronary artery bypass graft surgery is potentially a possibility. Advanced hemodynamic assessment and monitoring using a pulmonary artery catheterization may be taken into consideration in a subset of cardiac surgery patients (20).
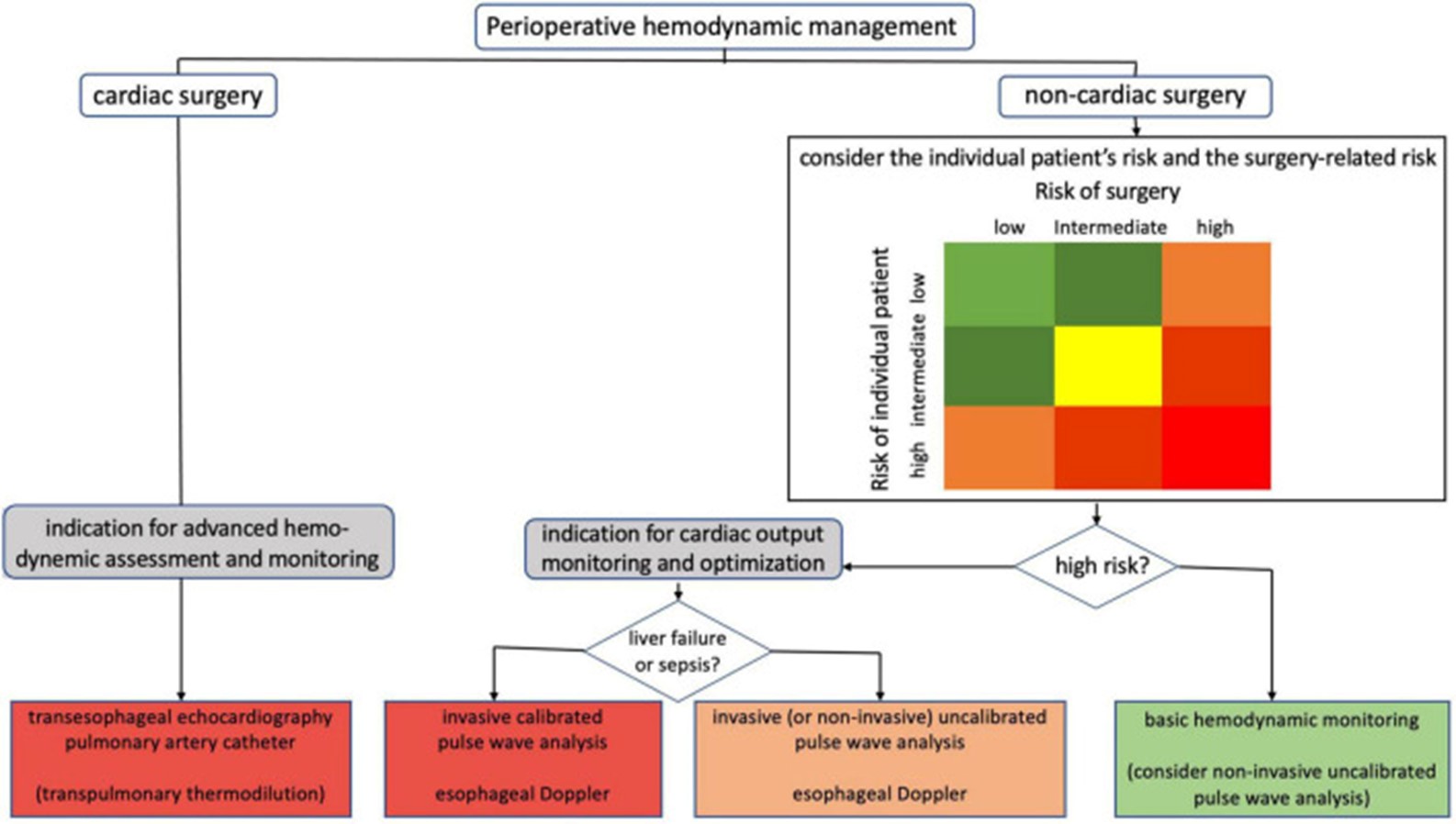
Figure 2: Indications for the various cardiac output evaluation techniques used during the perioperative hemodynamic care of surgical patients (20)
Fellahi et al. recommended that in conjunction with established methods for measuring cardiac output, which often entail invasive procedures, present challenges in usability and are evidently underutilized in the surgical setting, novel minimally invasive and non-invasive technologies, although not directly interchangeable with the former, hold promise in facilitating the incorporation of GDT and are likely to enhance outcomes in patients undergoing surgeries with moderate to high risk. Nonetheless, there exist exceptional situations where the application of invasive reference techniques is unavoidable, such as in cases of cardiac surgery or instances of shock. It is imperative for medical practitioners to recognize that, irrespective of the precision in estimating stroke volume, the pivotal aspect is to discontinue fluid administration promptly when stroke volume no longer exhibits an increase in response to fluid boluses (8). The algorithm for typical intraoperative goal-directed therapy based on a specialised approach is illustrated in Figure 3 (8). Our review signifies the importance of haemodynamic optimization strategies in high-risk surgical patients; however, to what extent these guidelines are practiced in surgical settings needs to be backed by further clinical research. Additionally, we could not assess the effects of these guidelines among patients based on outcomes including morbidity and mortality, which is the limitation of our paper; however, we tend to assess these outcomes in our future research studies as it is beyond the scope of this paper.
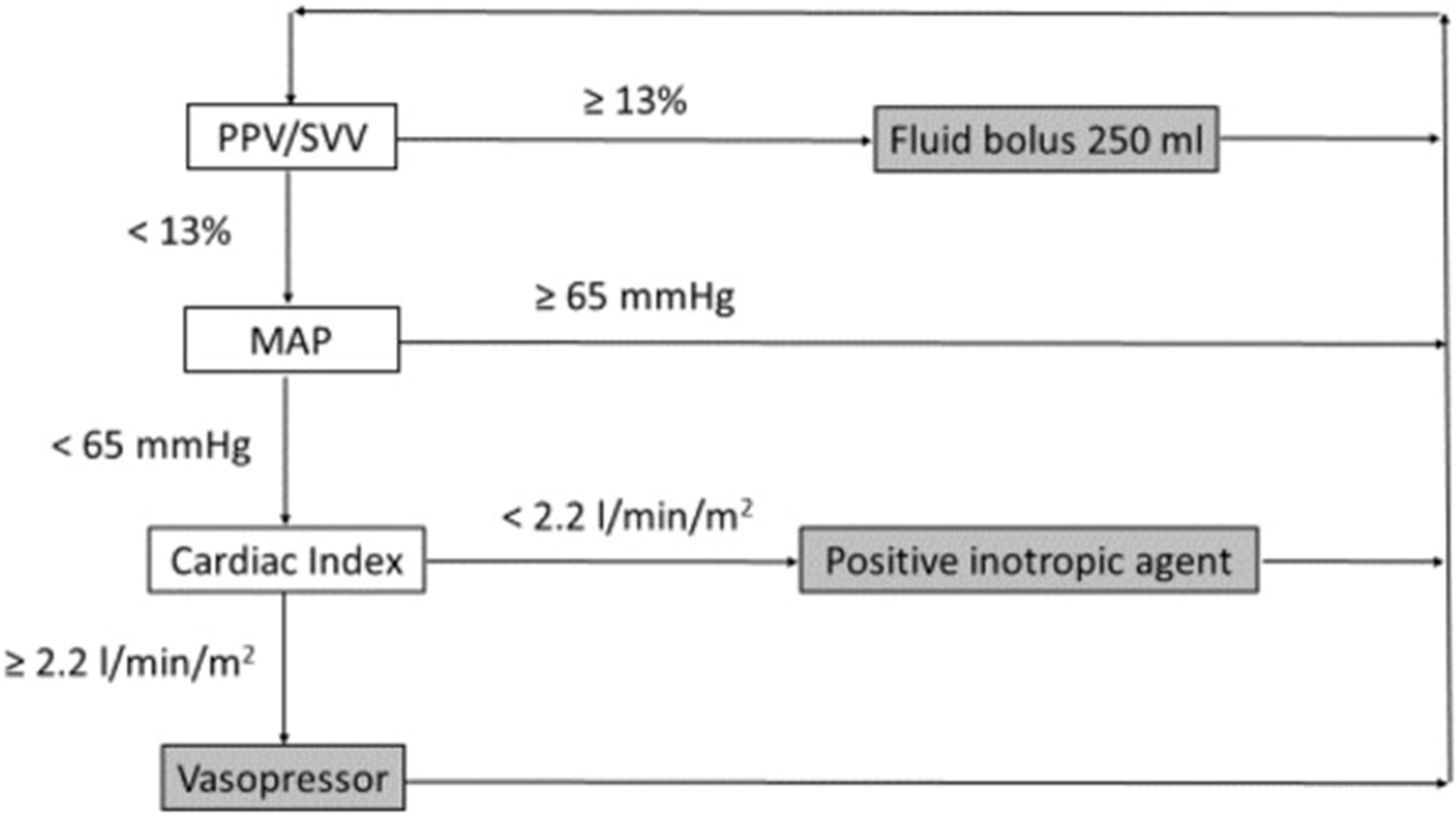
Figure 3: Algorithm for typical intraoperative goal-directed therapy based on a specialised approach (8)
Conclusion
Significant research evidence demonstrates that the employment of hemodynamic optimization strategies is highly beneficial for high-risk surgical patients since they reduce postoperative complications and may even enhance long-term outcomes when performed during the early stages of surgery. Haemodynamic optimization strategies, or GDT, are based on the administration of fluids, vasopressors, and inotropic medications. Only adherence to these strategies in clinical practice can aid in reducing morbidity and mortality in high-risk surgical patients.
Disclosure
Conflict of interest
There is no conflict of interest
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection, and final writing of the manuscript.
