Volume 3, Issue 6
June 2023
The Efficacy and Safety of Antispasmodic agents in the Management of Irritable Bowel Syndrome: A Systematic Review
Khalid Al Ghamdi, Naif Albluwi, Ali Alammari, Hassan Alibrahim, Amal Al-Thabet, Janan Radhi, Fahad Alnamshah, Khalid Alghamdi, Nashmeah Alshammari, Sahar Ghalib, Abdullah Al Dawood
DOI: http://dx.doi.org/10.52533/JOHS.2023.30602
Keywords: irritable bowel syndrome, antispasmodic agents, spasmolytic, efficacy, effectiveness, safety, adverse effects
Background: Irritable Bowel Syndrome (IBS) is a chronic gastrointestinal disorder with various subtypes and unclear causes. Antispasmodic agents, such as smooth muscle relaxants or anticholinergic drugs, target gut muscle contractions to relieve abdominal pain and spasms. Individualized treatment is important, considering symptoms, IBS subtype, and patient response. Combination therapy with antispasmodics, dietary adjustments, stress management, and probiotics can enhance symptom control and improve quality of life for IBS patients.
Methods: Online databases were searched for pertinent English publications that met the inclusion and exclusion criteria of this research (Cochrane library, PubMed, and web of science, respectively). Treatment response or therapeutic efficacy, global or clinical improvement, symptom reduction (abdominal pain relief, bowel motility, Bristol stool score), and adverse events (nausea, dizziness, high blood pressure) were all evaluated as clinical endpoints.
Results: Eight randomized clinical trials involving 1227 IBS patients were included. The studies were conducted between 2014 and 2020, with the majority of participants being female. The endpoints of the studies showed significant reductions in abdominal pain severity, bowel motility, ibs symptom severity, and improvements in quality of life and Bristol stool score. Adverse events were reported in all studies except one, with nausea and dizziness being the main findings. Overall, the review indicated varying degrees of effectiveness of antispasmodics in alleviating IBS symptoms.
Conclusion: In conclusion, antispasmodic agents, including mebeverine, otilonium bromide, alverine citrate/simethicone, pinaverium bromide, phloroglucinol, drotaverine hydrochloride, and peppermint oil, have shown effectiveness in relieving IBS symptoms. However, the quality and design of the studies varied, and many trials were short-term, limiting their clinical significance for a chronic condition like IBS. Further research with larger samples, longer durations, and standardized endpoints is needed to provide more conclusive evidence on the efficacy and safety of antispasmodics in managing IBS.
Introduction
Irritable bowel syndrome (IBS) is a chronic, functional gastrointestinal disorder characterized by abdominal pain, bloating, and altered bowel habits (1). It encompasses several subtypes, including IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and mixed IBS (IBS-M) (2). The exact cause of IBS remains unclear, but factors such as abnormal gut motility, visceral hypersensitivity, intestinal inflammation, and gut microbiota dysbiosis are thought to play a role in its pathogenesis (3, 4). It affects millions of individuals worldwide and significantly impacts their quality of life.
One approach to managing IBS symptoms involves the use of antispasmodic agents. These medications target smooth muscle contractions in the gut, providing relief from abdominal pain and spasms. Antispasmodic agents used in the management of IBS are classified as smooth muscle relaxants or anticholinergic drugs (5). These medications specifically target the neurotransmitter acetylcholine and work by inhibiting its effects on muscarinic receptors found in the smooth muscles of the gastrointestinal tract (6). Commonly prescribed antispasmodics for IBS include hyoscine butylbromide, dicyclomine, and mebeverine.
Hyoscine butylbromide, known as scopolamine butylbromide, selectively blocks muscarinic receptors in the gastrointestinal tract, effectively reducing visceral pain and smooth muscle spasms associated with IBS (7). Clinical trials have demonstrated its effectiveness in alleviating abdominal pain and discomfort, particularly in patients with IBS-D and IBS-M (8). The safety profile of hyoscine butylbromide is generally favorable, with limited penetration of the blood-brain barrier, leading to a low incidence of central nervous system side effects (9). Dicyclomine, a non-selective muscarinic antagonist, has been found to be effective in reducing abdominal pain, colonic spasm, and overall IBS symptoms. It acts by inhibiting smooth muscle contraction and reducing the sensitivity of visceral pain fibers (10). Clinical trials have shown its efficacy in different subtypes of IBS, including IBS-D and IBS-C. Adverse effects of dicyclomine, such as dry mouth, blurred vision, and constipation, are generally dose-related and reversible upon discontinuation (11). Mebeverine, which acts as a calcium channel blocker and exhibits anticholinergic effects, has also demonstrated efficacy in relieving abdominal pain, bloating, and overall IBS symptoms (12). Its mechanism of action involves reducing calcium influx into smooth muscle cells, resulting in relaxation of the gastrointestinal tract and alleviation of spasms (13). Mebeverine has a generally favorable safety profile, with a low incidence of adverse effects. Mild and transient gastrointestinal disturbances, including nausea and diarrhea, may occur (14). Individualized treatment approaches are crucial in selecting the most appropriate antispasmodic agent for IBS management. Factors such as the patient's specific symptoms, IBS subtype, and response to treatment should be taken into consideration (15). Some patients may respond better to a particular antispasmodic due to variations in receptor affinity, metabolism, or underlying pathophysiology (16). Combination therapy, involving antispasmodics along with dietary modifications, stress management techniques, and probiotics, may provide synergistic benefits and improve overall symptom control (17). While they may not address all symptoms, antispasmodic agents provide significant symptomatic relief and improve the quality of life for many IBS patients.
Methods
Definition of outcomes and inclusion criteria
Studies conducted on the efficacy and safety of antispasmodic agents in the management of IBS. The following clinical outcomes were reported by the studies: treatment response or therapeutic efficacy, global or clinical improvement, symptom reduction (such as relief of abdominal pain), and adverse events.
Search strategy
Online databases such as PubMed, Web of Science, and the Cochrane Library were searched for relevant articles that satisfied the pre-determined inclusion and exclusion criteria. The terms ‘irritable bowel syndrome’, ‘IBS’, ‘antispasmodic agents’, ‘antispasmodic’, ‘spasmolytic’, ‘efficacy’, ‘effectiveness’, ‘safety’, ‘adverse effects’ were used to find relevant publications. The phrases ‘alverine citrate’, ‘simethicone’, ‘mebeverine’, ‘otilonium bromide’, ‘otilonium’, ‘pinaverium’, ‘pinaverium bromide’ and ‘calcium channel blocker’ were also included one at a time in the search process in order to improve it. Reference lists of appropriate articles were also checked for pertinent publications.
Screening and extraction
Articles whose titles weren't pertinent were disqualified. The full text and abstracts of papers were examined in the second phase to include those that met the inclusion criteria. The titles and abstracts were organized, evaluated, and checked for duplicate entries using reference management (Endnote X8). We used a double screening technique to maintain outstanding quality throughout this key stage, with one screening used to analyze titles and abstracts and the other to assess entire texts. After ensuring that all significant articles were included, an organized extraction sheet that was relevant to our targeted outcomes was made. The page listed the desired outcomes as well as the baseline characteristics. Study design, sample size, and source were the baseline characteristics.
Quality assessment
We utilized the Jadad scoring method to review the methodological quality of the included trials (18). Studies are scored according to the presence of three key methodological features of clinical trials, specifically randomization, blinding, and accountability of all patients, including the loss to follow-up or withdrawal. One point is added for a “yes” answer to each of the first five items, and one point is subtracted for “yes” answer to either of the last two items, for an overall score of 0–5. The score ranges from 0 to 2, which indicates low quality, while 3 to 5 indicates high quality.
Results
Search results
We were able to uncover a total of 72 citations using the previously specified search techniques, which were subsequently reduced to 58 after duplicates were eliminated. Four studies were included after manually searching for relevant articles. Only 47 citations remained after the title and abstract screening and qualified for the following stages. Only eight articles met our inclusion and exclusion criteria after the full-text screening. Figure 1 displays the thorough search and screening procedure.
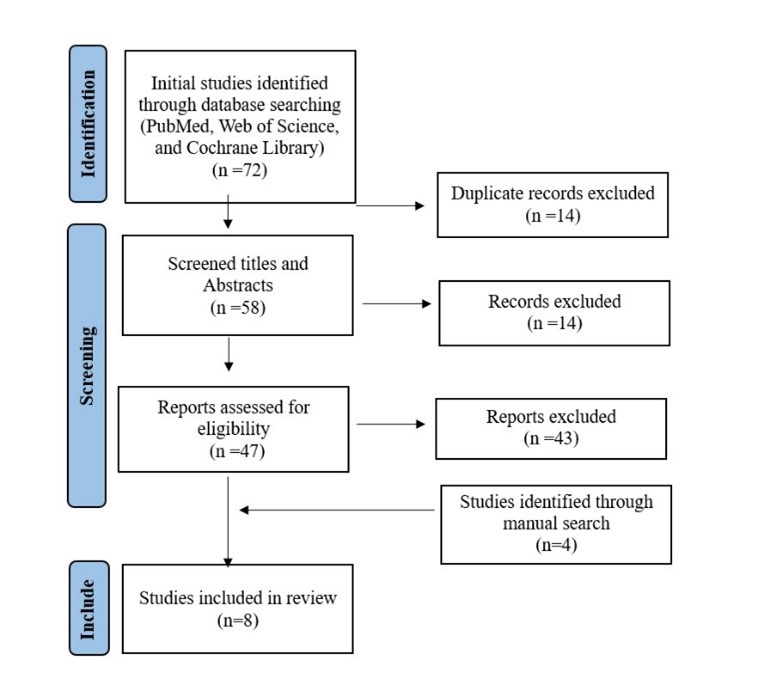
Figure 1: PRISMA flow diagram.
Results of quality assessment
All of the included studies had satisfactory quality and a low risk of bias, according to our assessment of bias. None of the listed research produced unacceptable outcomes (Table 1).
|
Table 1. Summary of the results of methodological quality assessment of the included studies using the Jadad scale |
||||||
|
Study |
Year |
Randomization |
Blinding |
Accounting for all patients |
Total modified Jaded score (maximum 3 points) |
Overall quality |
|
Chmielewska-Wilko? et al. (23) |
2014 |
2 |
1 |
1 |
4 |
High quality |
|
Ducrotte et al. (24) |
2014 |
2 |
2 |
1 |
5 |
High quality |
|
Rai et al. (22) |
2014 |
2 |
0 |
1 |
3 |
High quality |
|
Zheng et al. (21) |
2015 |
2 |
2 |
1 |
5 |
High quality |
|
Fan et al. (20) |
China |
2 |
2 |
1 |
5 |
High quality |
|
Chakraborty et al. (19) |
India |
2 |
2 |
1 |
5 |
High quality |
|
Shin et al. (32) |
Korea |
2 |
2 |
1 |
5 |
High quality |
|
Weerts et al. (34) |
The Netherlands |
2 |
2 |
1 |
5 |
High quality |
Characteristics of the study included
Finally, we examined eight studies that satisfied the eligibility criteria of this investigation and are included in this systematic review. The studies enrolled participants between 2014 and 2020, including 1227 clinically diagnosed IBS patients. The mean age of the included subjects ranged between 33.7 and 55.1 years. All of the included research investigations were randomized clinical trials. Regarding the countries covered by the included studies, India and China were the subjects of two studies each, while Italy, France, South Korea, and the Netherlands were each represented by a single study. Majority of the included IBS patients were females in all studies except one. The main characteristics of the included studies have been summarized in Table 2.
|
Table 2. Baseline characteristics of the included studies |
||||||
|
Study |
Country |
Year |
Study |
Total participants |
Mean Age (years) |
Gender (Female %) |
|
Chmielewska-Wilko? et al. (23) |
Italy |
2014 |
RCT |
93 |
44.8 ± 12.6 |
69% |
|
Ducrotte et al. (24) |
France |
2014 |
RCT |
436 |
Treatment: 53.8 (14.9) Control: 55.1 (16.2) |
73.40% |
|
Rai et al. (22) |
India |
2014 |
RCT |
180 |
Treatment: 45±18, Placebo: 48±16 |
50.00% |
|
Zheng et al. (21) |
China |
2015 |
RCT |
320 |
Treatment:36.9±11.8, Placebo: 36.6 ± 12.6 |
73.13% |
|
Fan et al. (20) |
China |
2017 |
RCT |
1044 |
36.47 |
57.47% |
|
Chakraborty et al. (19) |
India |
2019 |
RCT |
40 |
Treatment:33.65±9.60 Placebo: 37.6±11.09 |
67% |
|
Shin et al. (32) |
Korea |
2020 |
RCT |
72 |
Treatment:42.30±12.77, Placebo: 42.32 ± 12.80 |
63.90% |
|
Weerts et al. (34) |
Netherlands |
2020 |
RCT |
189 |
34.0 |
77.80% |
The major study endpoints, such as treatment response, symptom relief, IBS severity reduction, and adverse events, have been summarised in Tables 3A and 3B. Four included studies observed a significant reduction in abdominal pain severity (19-22), four studies observed a significant reduction in bowel motility (19, 20, 22, 23), three included studies found a significant reduction in IBS symptom severity (20, 21, 24) and two studies each reported significant improvements in IBS related quality of life (19, 24), global discomfort index (22, 23), and Bristol stool score (20, 21). All studies except one reported positive findings for adverse events. In the two studies that provided information on the type of adverse event, the main findings were nausea and dizziness. Overall results showed varying degrees of antispasmodic effectiveness in alleviating IBS symptoms.
|
Table 3A. Outcomes measures of included studies |
||||||
|
Study |
Antispasmodic |
Dosage |
Treatment response (Drug vs Placebo/Control) |
Symptom relief (Drug vs Placebo/Control) |
IBS severity reduction |
|
|
Drug |
Placebo/Control |
|||||
|
Chmielewska-Wilko? et al. (23) |
Otilonium |
20mg (n =24) 40mg (n= 23) 80 mg (n =23) |
NR |
Evacuation frequency, -41.9% vs -8.4% (p < 0.01) |
NR |
NR |
|
Ducrotte et al. (24) |
Phloroglucinol |
2.8 capsules/day |
58.60% vs 35.90% |
IBSQoL, Month 3: 74.9 (16.6) vs 68.8 (17.1) Month 6: 77.6 (16.4) vs 71.2 (18.4) At last follow-up, No abdominal pain: 36.9 vs 15.5 (P=0.0001) No bloating: 32.9 vs 10.6 (P< 0.0001) IBS-SSS < 75: 37.7 vs 16 (P< 0.0001) |
37.70% |
16.00% |
|
Rai et al. (22) |
Drotaverine |
80 mg |
NR |
Global abdominal pain relief, Patient-reported: 85.9% vs 39.5% (P<0.01) Clinician-reported: 82.4% vs 36.5% (P<0.01) |
77.70% |
30.60% |
|
Zheng et al. (21) |
Pinaverium |
50 mg |
Week 2: 50% vs 22% Week 4: 77.5% vs 33.5% |
Abdominal pain, Week 2: 40.4% vs 16.7% (P<0.001) Week 4: 62.4% vs 29.7% (P<0.001) Stool consistency, Week 2: 22.9% vs 11.5% (P<0.005) Week 4: 53.2% vs 20.6% (P<0.001) |
NR |
NR |
|
Fan et al. (20) |
Pinaverium |
50 mg |
58.3% vs 21.8% |
Abdominal pain, Week 2: 33.3% vs 47.7% (P<0.001) Week 4: 27.6% vs 51.4% (P<0.001) Stool consistency, Week 2: 72.1% vs 30.7% (P<0.005) Week 4: 75.6% vs 50.6% (P<0.001) |
NR |
NR |
|
Chakraborty et al. (19) |
Mebeverine |
200 mg, 2 times /8 weeks |
0 |
Daily bowel movements in past 7 days, n, Week 4: 4.3±2.18 vs 4.2 ± 1.30 (P=0.766) Week 8: 3.8 ±2.34 vs 3.8 ± 1.51 (P=0.756) Abdominal pain, Week 2: 1.4 ± 0.59 vs 1.6 ± 0.75 (P=0.482) Week 4: 1.3 ± 0.57 vs 1.5 ± 0.75 (?P=0.615) |
5.0, Week 4 :4.0, Week 8: 3.5 |
5.5, Week 4: 4.0 Week 8: 4.0 |
|
Shin et al. (32) |
Phloroglucinol |
80 mg |
Week 3: 61.6% vs 30.60% |
Passage of gas at Week 2, 4.00 ± 1.93 vs 4.23 ± 1.87 (P=0.016) Stool frequency, 2.01 ± 0.85 vs 1.54 ± 0.94 (P=0.090) |
73.17± 18.26 |
78.41±16.6 |
|
Weerts et al. (34) |
Peppermint oil |
182 mg |
NR |
SPO vs IPO vs placebo, Abdominal pain (FDA standard, at least 30% decrease in mean worst daily abdominal pain in at least 4 out of 8 weeks: 29 (46.8) vs 26 (41.3) vs 22 (34.4) (P= 0.170) (P= 0.385) Global relief: 6 (9.7) vs 1 (1.6) vs 3 (4.7) (P= 0.317) (P= 0.351) Abdominal pain (at least 50% decrease in mean worst daily abdominal pain in at least 4 out of 8 weeks): 16 (25.8) vs 13 (20.6) vs 8 (12.5) (P= 0.062) (P= 0.220)
|
1:277.0±73.6 2:281.8±68.7 |
270.8± 74.2 |
NR, Not reported; IBSQoL, IBS-related Quality of Life score; IBS-SSS, IBS-severity symptom score; SPO, Small-intestinal–release peppermint oil; IPO, Ileocolonic-release peppermint oil.
Discussion
Investigators have hypothesized that intestinal motility issues cause abdominal cramps, bloating, and erratic defecation, even though the exact pathophysiology of IBS is still unknown (25) (26). Mebeverine and pinaverine, for example, directly alter the relaxation of intestinal smooth muscle, while dicyclomine and hyoscyamine functioning similarly possess anticholinergic or antimuscarinic characteristics (27). Antispasmodics most likely work by reducing the naturally occurring activity of the intestinal smooth muscle.
|
Table 3B. Outcomes measures of included studies |
|||||||||||||||||||
|
Study |
Total adverse events |
Nausea |
Dizziness |
Increased blood pressure |
Abdominal discomfort |
||||||||||||||
|
Antispasmodic vs Placebo/Control |
|||||||||||||||||||
|
Chmielewska-Wilko? et al. (23) |
5% |
NR |
NR |
NR |
NR |
||||||||||||||
|
Ducrotte et al. (24) |
2.2% vs 0 |
0 vs 0 |
0 vs 0 |
0 vs 0 |
0 vs 0 |
||||||||||||||
|
Rai et al. (22) |
4% vs 3% |
1 vs 2 |
1 vs 0 |
0 vs 0 |
|||||||||||||||
|
Zheng et al. (21) |
3.7% vs 1.9% |
3.2% vs 0.5% |
2.3% vs 1% |
2.3% vs 1% |
|||||||||||||||
|
Fan et al. (20) |
18.7% vs 14.7% |
2.9% vs 1.7% |
2% vs 3.2% |
2.3% vs 0.9% |
2.9% vs 0.9% |
||||||||||||||
|
Chakraborty et al. (19) |
NR |
NR |
NR |
NR |
NR |
||||||||||||||
|
Shin et al. (32) |
5.6% |
NR |
NR |
NR |
NR |
||||||||||||||
|
Weerts et al. (34) |
1.6% |
35.9% |
NR |
NR |
NR |
||||||||||||||
NR, Not reported.
Mebeverine
Chakrobarty et al. conducted a randomized trial with a double-blind, placebo-controlled design to assess the effectiveness and safety of mebeverine (19). One group received mebeverine 200 mg controlled-release tablets twice daily for 8 weeks, while the other group received a matching placebo. The study measured various outcomes, including the number of bowel movements per day over the past 7 days (NoBM7d), severity of abdominal cramps, and the IBS quality of life (IBSQoL) score. Medication adherence and treatment-emergent adverse events were also recorded. The mebeverine group showed a modest but statistically significant improvement in NoBM7d (5.6±2.06 vs. 4.3±2.18 vs. 3.9±2.34; p < 0.001), cramps (1.8±0.69 vs. 1.4±0.59 vs. 1.3±0.57; p < 0.001), and IBSQoL compared to baseline at both 4 and 8 weeks. Statistical significance was absent in changes within the placebo group, and between the two groups at 4 and 8 weeks. Adherence to the treatment was better in the mebeverine group, and both interventions were well tolerated. The authors concluded that mebeverine 200 mg controlled-release tablets twice daily had a modest effect on IBS-D symptoms and may not be the optimal choice for patients with severe symptoms.
Daniluk et al. conducted a systematic review in 2022 to assess the efficacy and safety of mebeverine therapy for IBS (28). Their analysis inferred that mebeverine is an effective treatment option. Among the 22 studies included, which comprised both experimental and observational studies, six studies (including the clinical trial conducted by Chakrobarty et al.) reported a significant decrease in abdominal pain after mebeverine treatment (p-values ranging from <0.05 to <0.001). Only three studies did not show improvement in the severity of abdominal pain or discomfort with mebeverine treatment. Some of the included studies also demonstrated significant improvements in abnormal bowel habits, abdominal distension, stool frequency, and consistency. Adverse events associated with mebeverine were rare and mainly related to IBS symptoms.
Otilonium bromide
Chmielewska-Wilko? et al. conducted a clinical study on patients with treatment-sensitive functional IBS to assess the efficacy and safety of otilonium bromide (OB) (23). The study followed a double-blind, placebo-controlled, and randomized design, with patients assigned to four parallel groups. They received the drug at different doses or a placebo for a month. While individual parameters such as abdominal discomfort, bloating, and pain showed reductions with OB treatment over the 4-week period, no statistically significant differences were observed between the treatment groups. Additionally, there was no significant distinction between OB treatment and placebo in relation to mucus in the stool or incomplete or difficult evacuation. However, the frequency of evacuations significantly decreased after 4 weeks of treatment with the highest dose compared to placebo (-41.9% vs. -8.36%; p < 0.01). Although 21.7% of participants in the placebo group developed normal intestinal habits after 4 weeks, the improvement was more pronounced in the 40 mg OB group (p < 0.01). Furthermore, there was a dose-dependent decrease in the frequency of diarrhea (χ2-test for trend = 11.5; p < 0.001) and a rise in normal stool frequency. Combining the parameters into a global discomfort index showed a significant improvement with increasing doses of OB. No significant difference in the occurrence of adverse events was found between the placebo and OB groups. The investigators concluded that OB therapy at doses of 40 mg and 80 mg can effectively alleviate IBS manifestations compared to placebo over a 4-week period.
In a systematic review conducted by Heading et al. in 2005, which assessed the safety and tolerability of pharmaceutical agents used for IBS, adverse events were reported in four out of six trials that investigated otilonium bromide (29). Among these studies, two reported positive findings for adverse events. One trial reported minor nausea, while the other documented a patient's withdrawal due to drug intolerance.
Alverine citrate/simethicone
Ducrotte et al. conducted a comprehensive study employing a randomized, double-blind, placebo-controlled design to compare the efficacy of on-demand alverine citrate/simethicone (ACS) combination treatment with conventional antispasmodics for alleviating symptoms of IBS (24). To recruit participants, Rome III IBS patients were enrolled through the involvement of 87 general practitioners, and they were randomly assigned to either the on-demand ACS treatment or the conventional antispasmodics prescribed by their respective physicians. The primary objective was to assess the improvement in the IBS quality of life (IBSQoL) score from the baseline to the end of the 6-month study period. The analysis revealed a significantly greater improvement in the IBSQoL score in the ACS group compared to the usual treatment group (13.8 vs. 8.4; p < 0.0008). The ACS group also demonstrated lower scores on the IBS-severity symptom score (IBS-SSS) compared to the usual treatment group, indicating a mean decrease of 170.0 (SE 6.6) vs. 110.7 (SE 6.7) respectively (p = 0.0001). Moreover, a higher proportion of patients in the ACS group achieved an IBS-SSS score below 75 compared to the usual treatment group (37.7% vs. 16.0%; p < 0.0001). Notably, the on-demand ACS treatment exhibited greater improvements in abdominal pain and bloating severity and was associated with reduced direct and indirect costs. Based on these compelling findings, the investigators concluded that the on-demand ACS treatment, after 6 months, led to substantial enhancements in the quality of life, reduced disease burden, and was more effective in relieving symptoms of IBS compared to conventional treatments.
In a comprehensive systematic review and meta-analysis conducted by Martínez-Vázquez et al. in 2012, the clinical effectiveness of antispasmodics available in Mexico was thoroughly evaluated (30). The authors made an interesting observation that the addition of simethicone not only enhanced the properties of alverine but also exhibited a similar effect with pinaverium. Additionally, using the Peto method for patient global assessment, they detected a significant difference in favor of the alverine/simethicone combination (OR 2.03, 95% CI 1.49-2.77) and otilonium (OR 1.76, 95% CI 1.18-2.61) compared to eight other monotherapies and the pinaverium/simethicone combination.
Tongxie and Pinaverium bromide
Fan et al. conducted a well-designed randomized controlled trial to investigate the effectiveness of tongxie, a herbal formulation, in comparison to a placebo or pinaverium for alleviating symptoms of IBS (20). Rome III IBS patients were enrolled in the study and randomly assigned to one of three treatment groups: tongxie, placebo, or pinaverium bromide. The tongxie group received a combination of specific herbs tailored to individual patient features, including A. macrocephalae, P. lactiflora, C. reticulata, S. divaricata, C. pilosula, C. wenyujin, C. medica, and P. cocos, along with other herbs. The therapy course was 4 weeks long, during which the main outcomes were aimed at significant decreases in abdominal pain and Bristol stool score in patients administered tongxie in comparison to those being administered placebo or pinaverium. Other outcomes included a decrease in pain and stool frequency, as well as abdominal discomfort and its occurrence. The results revealed that patients receiving tongxie experienced significant reductions in all six assessed symptoms compared to those receiving placebo (P < 0.001). Moreover, a higher proportion of tongxie-treated patients reported increased stool consistency (75.6% vs. 50.6%), and a higher proportion had a decrease in daily stools (72.7% vs. 58.3%) (P < 0.001 for both). However, patients receiving pinaverium had significantly higher proportions reporting reduced pain (63.5%) and pain frequency (69.5%) compared to the tongxie group (51.4% and 58.6%, respectively; P < 0.005 for both). Based on these findings, the investigators concluded that a 4-week treatment course of tongxie could lead to significantly greater symptom reduction compared to placebo, as well as improvements in stool consistency and reductions in stool frequency compared to pinaverium. Consequently, tongxie presents itself as a promising alternative therapy for IBS patients who do not respond well to conventional treatments.
Zheng et al. conducted a meticulous and comprehensive prospective study using a double-blind, placebo-controlled design to examine the efficacy and safety of pinaverium in the management of IBS (21). According to Rome III criteria, patients were randomly allocated to two groups: one receiving pinaverium (50 mg, three times a day) and the other receiving placebo (three times a day). The primary objectives were centered around evaluating reductions in abdominal pain and Bristol stool score, while the secondary objectives focused on assessing changes in pain and stool frequencies, abdominal discomfort, and its frequency. Additionally, the study examined alterations in IBS global symptom scores and the occurrence of any adverse effects. A significantly higher number of patients in the pinaverium group achieved the primary outcomes, with 50.0% at week 2 and 77.5% at week 4, compared to the placebo group (P < 0.001). Analysis of symptom scores demonstrated a significantly higher percentage of patients experiencing improvement in their IBS symptoms in the pinaverium group (60%) compared to the placebo group (34%; P < 0.001). Therapy was well-tolerated and did not elicit severe adverse effects. Commonly reported side effects included mild occurrences of nausea (3.7%), dizziness (3.2%), and elevated blood pressure (2.3%). Based on these findings, the investigators confidently concluded that pinaverium effectively alleviates IBS symptoms and should be regarded as a viable first-line treatment option.
Bor et al. conducted a meticulous systematic review and meta-analysis involving eight placebo-controlled trials to examine the effectiveness of pinaverium bromide in IBS (31). The analysis revealed positive outcomes, indicating that pinaverium treatment significantly relieved overall IBS symptoms, as evidenced by a favorable standardized mean difference of 0.64 (95% CI 0.45-0.82; p < 0.0001) and a positive risk ratio of 1.75 (1.26-2.43; p < 0.0008). The odds ratio was calculated to be 3.43 (2.00-5.88; p < 0.0001), and the number needed to treat (NNT) was 4. Pinaverium also exhibited notable benefits in alleviating abdominal pain, normalizing stool patterns, and improving or resolving bloating. Remarkably, this meta-analysis underscored the significantly lower NNT for antispasmodics versus placebo in IBS studies and meta-analyses compared to other treatment approaches.
Phloroglucinol
Shin et al. conducted a comprehensive study to assess the effectiveness and safety of phloroglucinol in patients diagnosed with IBS-D. Rome III criteria, patients for IBS-D were assigned randomly in a parallel and double-blind manner to receive either phloroglucinol or placebo for a 2-week period (32). Following the treatment, the patients were monitored for an additional week. The main aim was to determine the percentage of participants, defined as those reporting "moderate or more improvement" in the patient global assessment for at least a week during the half month treatment period. Although the phloroglucinol group exhibited a higher proportion of responders during the 2-week treatment period compared to the placebo group, the difference did not reach statistical significance (55.6% vs. 30.6%, P = 0.056). However, during the 3-week period, the phloroglucinol group demonstrated a significantly higher proportion of responders compared to the placebo group (61.6% vs. 30.6%, P = 0.013). No serious adverse events were reported in either group. The authors found it to represent a safe and beneficial option for managing IBS-D manifestations.
In placebo-controlled trials conducted by Chassany et al., the efficacy of phloroglucinol and its methylated derivative (TMP) was investigated in patients with unspecified IBS. They observed a relative reduction in pain intensity of 57.8±31.7% for phloroglucinol and 46.3±34.7% for TMP on day 7, and the percentage of patients experiencing at least a 50% decrease in pain intensity was 62.3% for phloroglucinol and 47.0% for TMP (15.3±5.7% [CI95% 4.1-26.5]). They concluded that a one-week treatment with phloroglucinol/TMP significantly alleviates pain intensity in patients with IBS.
Drotaverine hydrochloride
Rai et al. conducted a double-blind, placebo-controlled parallel group study to evaluate the effectiveness and safety of drotaverine hydrochloride (HCl) in providing relief to patients with IBS (22). The study involved randomizing patients who met the criteria outlined in the Rome II Criteria to receive either drotaverine or a placebo. Throughout the four-week treatment period, the researchers assessed abdominal pain and stool frequency every week in both groups. They evaluated the Subject Global Assessment of Relief (SGA) to study the overall improvement in IBS symptoms at the conclusion of the trial. The results indicated a significant reduction in pain frequency in the drotaverine group, with 25.9%, 60%, and 77.7% of patients experiencing a decrease at the end of the 2nd, 3rd, and 4th weeks, respectively, compared to 9.4%, 21.2%, and 30.6% in the placebo group. Furthermore, pain severity scores significantly decreased in the drotaverine group, with 77.7% of patients experiencing improvement after four weeks, compared to 30.6% in the placebo group. The drotaverine group also demonstrated significant improvement in global relief of abdominal pain, as reported by both patients (85.9% vs. 39.5%) and clinicians (82.4% vs. 36.5%), in contrast to the placebo. Evacuation occurrence also improved significantly in the drotaverine HCl treatment group compared to the placebo. Overall, drotaverine was well-tolerated, with no major side effects reported. The researchers concluded that a four-week treatment with drotaverine significantly improved abdominal symptoms in patients with IBS.
Xue et al. conducted a placebo-controlled trial to evaluate the effectiveness and safety of drotaverine hydrochloride in Chinese patients with IBS (33). The study revealed significant improvements in the treatment group compared to the placebo group after 4 weeks. Specifically, the assessment of abdominal pain on the visual analog scale showed better results (1.8 [0.7] vs. 4.4 [1.5]; P < 0.01), as well as improvements in stool frequency (1.4 [0.6] vs. 2.9 [0.9]; P < 0.01) and Bristol score (5.1 [0.6] vs. 5.6 [1.0]; P < 0.01). However, there was no significant difference observed in the quality of life measured using the 36-item short form health survey, and no notable adverse events were reported.
Peppermint oil
Weerts et al. conducted a double-blind trial to examine the efficacy and safety of small-intestinal-release peppermint oil (SPO) and targeted ileocolonic-release (IPO) in patients with IBS (34). Rome IV criteria were randomly assigned to receive SPO, IPO, or placebo for 8 weeks. The main outcome was the response in abdominal pain, defined as a minimum 30% decrease in the weekly average of the worst daily abdominal pain compared to baseline for at least 4 weeks (P = 0.170 for SPO vs. placebo; P = 0.385 for IPO vs. placebo). The other main outcome included was overall relief of IBS symptoms, according to the European Medicines Agency definition (P = 0.317 for SPO vs. placebo; P = 0.351 for IPO vs. placebo). Secondary outcomes assessed included abdominal pain, discomfort, symptom severity, and adverse events. The study found no significant differences in abdominal pain response between the drug and placebo groups. There were also no significant differences in overall symptom relief among the groups. However, the SPO group showed greater improvements in secondary endpoints such as abdominal pain (P = 0.016), discomfort (P = 0.020), and IBS severity (P = 0.020) compared to the placebo group. Adverse events, although mild, were more common in both drug groups (P < 0.005). The investigators concluded that neither drug group demonstrated significant reductions in abdominal pain response or overall symptomatic relief based on the recommended outcomes. However, the SPO did show significant improvements in abdominal pain, discomfort, and IBS severity. Therefore, the study did not advise more investigations into IPO for IBS management.
Khanna et al. conducted a comprehensive review and meta-analysis to assess the effectiveness and safety of enteric-coated capsules containing peppermint oil in comparison to placebo for the treatment of active IBS. The findings revealed that peppermint oil demonstrated a significant advantage over placebo in terms of improving overall IBS symptoms (relative risk 2.23; 95% CI 1.78-2.81) and alleviating abdominal pain (relative risk 2.14; 95% CI 1.64-2.79). The analysis also indicated that although patients receiving peppermint oil reported a higher incidence of adverse events, these events were generally mild and temporary in nature. Among the reported adverse events, heartburn was the most commonly encountered (35).
Strength and limitation
To the best of our knowledge, this is the first updated comprehensive analysis of the efficacy and safety of antispasmodic agents in the management of IBS since 2013. The fact that there was significant heterogeneity among the research endpoints that were included may be a limitation. Further, most studies were conducted on a small sample size, and for durations under eight weeks. Exploratory secondary endpoint analyses are subject to possible power limitations and increased type I error, and short-term clinical trials assessing the efficacy of treatments for IBS are deemed to have limited clinical significance due to the chronic and recurring nature of the condition. Another limitation refers to the quality of the included studies, which also varied. For more conclusive evidence, more studies with similar endpoints are needed. Not all of the included studies contained information on aspects including ethnicity as well as related issues such as comorbidities, types of IBS, and dietary factors.
Conclusion
Antispasmodic agents are a commonly used approach for managing the symptoms of IBS. Mebeverine, otilonium bromide, alverine citrate/simethicone, pinaverium bromide, phloroglucinol, drotaverine hydrochloride, and peppermint oil have shown varying degrees of effectiveness in alleviating IBS symptoms. These medications target smooth muscle contractions in the gut, providing relief from abdominal pain and spasms. However, the overall quality of evidence and study design varied across the included studies, and the short-term nature of many trials may limit their clinical relevance for a chronic condition like IBS. Further research with larger sample sizes, longer durations, and standardized endpoints is needed to provide more conclusive evidence on the efficacy and safety of antispasmodics in managing IBS.
Disclosure
Conflict of interest
There is no conflict of interest
Funding
No funding
Ethical consideration
Non applicable
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.