Volume 6, Issue 1
January 2026
Effect of Long-Term Immunosuppressive Therapy on Soft Tissue and Bone Stability
Maha Assad Munshi, Nouf Abdullah Mansour, Raghad Khalid Alsaeed, Faris Mohammed Alshakweer, Manal Abdulrhman Daish, Razan Yasser Alauti, Abdulmalik Marwan Khateeb, Sameer Saleh Alzahrani, Abdulaziz Awwadh Alqurayqiri, Tala Muhammad Hotah
DOI: http://dx.doi.org/10.52533/JOHS.2026.60108
Keywords: Immunosuppressive drugs, periodontal tissue, bone stability, organ transplant, gingival overgrowth
Immunosuppressive drugs are essential for managing autoimmune diseases and preventing organ transplant rejection. However, their long-term use has significant adverse effects on various physiological systems, including the oral cavity. Immunosuppressive agents, particularly corticosteroids, cyclosporine, and mycophenolate mofetil, alter the oral microbiome, promoting the colonization of opportunistic pathogens such as Pseudomonas aeruginosa and Staphylococcus aureus. These microbial changes increase the risk of candidiasis, periodontitis, and gingival inflammation. Additionally, cyclosporine is strongly associated with gingival overgrowth, affecting approximately 30% of patients, with severe cases requiring surgical intervention.
Beyond soft tissue effects, immunosuppressive therapy also compromises bone stability. Corticosteroids disrupt calcium metabolism, leading to decreased bone mineral density (BMD), increased fracture risk, and osteoporosis, particularly in the maxilla and mandible. The degree of bone loss correlates with dosage and duration of corticosteroid use, with trabecular bone being more susceptible than cortical bone. The reduction in BMD contributes to tooth loss and alveolar bone resorption, worsening oral health outcomes.
Despite advancements in immunosuppressive therapy, effective strategies to mitigate its detrimental effects on oral tissues remain limited. Regular dental monitoring, dietary interventions rich in calcium and vitamin D, and personalized immunosuppressive regimens may help reduce oral complications. Further research is needed to develop targeted therapies that minimize immunosuppressive drug-induced damage while maintaining therapeutic efficacy. This review article explores the impact of immunosuppressive therapy on the stability of soft tissues and bone within the oral environment. It also highlights the importance of interdisciplinary collaboration between medical and dental professionals to ensure comprehensive care for patients undergoing long-term immunosuppressive therapy.
Introduction
Immunosuppressive drugs are drugs that reduce the human body’s normal immune response, suppress the immune system, or prevent the production of antibodies (1). Immunosuppressant drugs are used to prevent, treat, or alleviate symptoms of autoimmune diseases such as myasthenia gravis, glomerulonephritis, arthritis, lupus, rheumatoid arthritis, Crohn’s disease, and organ transplant rejections (2) such as liver transplant, kidney transplant and heart transplant (1). Organ transplant rejection can either be acute, if the rejection occurs immediately after the transplant, or chronic, if the rejection occurs after a long period. Immunosuppressive drugs prevent the production of the antibodies that attack the transplanted organ (1). The commonly used immunosuppressive drugs include corticosteroids, sirolimus, azathioprine, cyclosporine A, and tacrolimus (1). The dosage of immunosuppressive drugs depends on the immune status of the patient, age, sex, nutrition, the purpose of using the drug, and combination with other immunosuppressive drug (1). The dosage also depends on a certain protocol. For instance, if the drug is used to suppress a predominant immune effector or resolve an acute inflammation, a high dosage is used. However, if the immunosuppressive agent is used to prevent relapse of a certain disease, then a low dose of the drug is used for long term (3).
Corticosteroids are the most used immunosuppressive drugs. They inhibit mainly cell-mediated immunity by binding to the steroid receptor and changing the transcription of genes. Hence, it inhibits the production of antibodies and complements in plasma and inhibits the transcription of cytokines as well (1). They are used in bone marrow transplantation, systemic lupus, rheumatoid arthritis, Inflammatory eye disease (IED) (4), inflammatory bowel condition, systemic dermato-myositis, psoriasis, asthma, autoimmune hematological disorders, and suppress inflammation associated with organ transplant rejection (1). However, their widespread use of treating several diseases has caused serious adverse effects such as severe toxicity, diabetes, hypertension, infection, Cushing’s syndrome, osteoporosis, accelerated cardiovascular disease, poor wound healing, growth retardation, osteopenia, Osteoporosis, avascular necrosis of bone, hyperglycemia, fluid retention, cataract and decreased patients' capacity to synthesize corticosteroids (1, 5). As a result, corticosteroids are discontinued after a few weeks or months (5). This explains why most patients are free of steroids after just six months (6). Cyclosporine A is another immunosuppressive drug that is prescribed to prevent acute rejection and inhibit the replication of the Hepatitis C virus in transplantation (7-9) and treat some renal diseases such as nephrotic syndrome (1). It inhibits the immune system by inhibiting signal transduction in T-lymphocytes (1). In recent years, new immunosuppressive agents have been developed. For instance, azathioprine, which was a mainstay of immunosuppression (6), was proven to cause renal toxicity (10, 11). Therefore, it was replaced with a more potent and less toxic immunosuppressive drug called Mycophenolate mofetil (12).
Despite recent advancements in the development of immunosuppressive agents, these medications remain associated with significant side effects. Their non-selective nature results in unintended impacts on various physiological systems (1), including the oral cavity. Research indicates that immunosuppressive drugs can compromise the stability of both soft tissues and bone within the oral environment. This review article aims to discuss the effect of immunosuppressive drugs on the stability of soft tissues and bone in the oral cavity.
Methodology
This narrative review is based on a comprehensive literature search conducted on March 9, 2025, using Dynamed, ScienceDirect, PubMed, Wiley Library, MDPI, Oxford Academic, BMC, and Cochrane databases. The research utilized Medical Subject Headings (MeSH) terms and relevant keywords, such as the effect of long-term immunosuppressive drugs on the oral cavity, to identify studies that examined the impact of immunosuppressive therapy on soft tissue and bone stability. A manual search was also conducted using Google Scholar, and the reference lists of identified papers were reviewed to locate additional relevant studies. No restrictions were applied regarding publication date, language, participant age, or type of publication, ensuring a broad and inclusive exploration of the available literature.
Discussion
Effects on Soft Tissue Stability
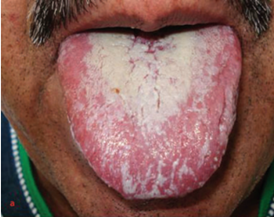
Immunosuppressive agents negatively affect the health of the oral cavity. Organ transplant recipients take immunosuppressive drugs to prevent rejection of the transplanted organ. However, immunosuppressive drugs inhibit the immune system, hence, they change the oral microbe (13). In immunocompromised and hospitalised patients, the oral cavity is colonised by Gram-negative bacteria such as Pseudomonas aeruginosa, Pseudomonas fluorescens, Klebsiella pneumoniae, and Acinetobacter baumannii and by Gram-positive bacteria such as Staphylococcus aureus and Enterococcus faecalis (13). The colonisation of the oral cavity by these pathogens was attributed to the decreased salivary flow, antibiotic use, and the lack of oral hygiene in addition to the immunosuppressive agents (13). Diaz et al. conducted a study in which the patients did not receive antibiotics, had good oral hygiene, and had good oral health (13). After excluding all potential confounding factors, the oral cavities of the subjects remained colonised by these pathogens. This finding underscores the role of immunosuppressive agents in amplifying the frequency of bacterial colonisation in the oral cavity (13). Additionally, there is a direct relation between the dose of immunosuppressive drugs and the number of opportunistic pathogens present in the oral cavity (14). However, we must take into consideration that immunosuppressant regimes often consist of two immunosuppressive agents. For instance, prednisone and mycophenolate mofetil are one of the most common combinations of immunosuppressants. These two agents are strongly correlated with the abundance and the prevalence of opportunistic pathogens in the oral cavity due to their effect on the oral microbiome (14). However, prednisone showed a stronger correlation than mycophenolate mofetil, underscoring the significant effect of prednisone on the bacterial colonisation in the oral cavity (14). Corticosteroids alter the oral microbiome balance by promoting the growth of certain pathogenic bacterial species and reducing microbial diversity. This promotes gingival inflammation, periodontitis and candidiasis (Figure 1) (15). In contrast, Mycophenolate mofetil is strongly correlated with Candida species load in the oral mucosa, but there was no correlation between it and Candida albicans (16).
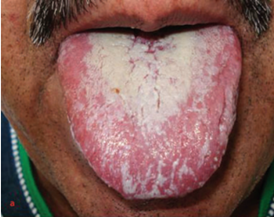
Figure 1: Candidiasis due to prolonged use of immunosuppressive agents (17).
Immunosuppressive agents affect periodontal tissues. Been et al. conducted a study on renal transplant recipients before the transplantation procedure and after it for up to six years (18). They found that the rate of periodontal breakdown and the severity of gingival bleeding were not affected by the transplantation procedure or the immunosuppressive agents (18). The inhibitory effect of immunosuppressive therapy decreases the response of the periodontal tissues to inflammation (18). On the contrary, another study stated that immunosuppressive agents decrease gingival inflammation but do not affect the rate of bone loss or attachment loss (18, 19).
The long-term administration of Cyclosporin results in gingival overgrowth (Figure 2). About 30% of dentulous patients who receive cyclosporine develop significant gingival overgrowth that may require treatment (20, 21). In edentulous patients, denture trauma and candidal infections participate in the gingival overgrowth (19). Additionally, 50% of the patients who receive a combination of Cyclosporine and Nifedipine experience gingival overgrowth (19). It is projected that approximately one billion patients are anticipated to be prescribed Cyclosporine within the next decade. Among these patients, it is estimated that 30% will develop gingival overgrowth, a condition that may necessitate surgical intervention. Moreover, of those experiencing this adverse effect, it is expected that half will require multiple surgical corrections throughout their treatment journey (19). Gingival overgrowth starts to appear after three months of receiving the drug (22). The labial gingiva is the most affected part of the gingiva (22). In addition to the pharmacological effects of Cyclosporine and Nifedipine, several other factors play a significant role in the development of gingival overgrowth. These factors include the presence of plaque-induced inflammation, individual genetic predispositions, and the inherent susceptibility of gingival fibroblasts. Each of these contributors interacts synergistically, exacerbating the condition and complicating its management (23). The management of gingival overgrowth includes discontinuing the drug causing it, reducing the dose of the drug without discontinuing it, and surgical correction in severe cases (23). In addition to administrating Metronidazole 400 mg for seven days to resolve the Cyclosporine-induced gingival overgrowth (24). Metronidazole has an antibacterial effect on the gingival plaque; hence, it reduces gingival inflammation and fibrous enlargement. However, Metronidazole increases the serum concentrations of Cyclosporine, which can lead to renal damage (19). In susceptible patients, good measures of oral hygiene and plaque removal improve patients’ gingival health, however, it does not prevent the gingival overgrowth (25).
Immunosuppressive agents, notably corticosteroids, are known to significantly impede the process of wound healing within the oral cavity. Specifically, patients undergoing long-term corticosteroid therapy often experience prolonged healing times following dental extractions. This delay can be attributed to the drug's adverse effects on various physiological processes, including the inhibition of normal tissue repair mechanisms, angiogenesis, cellular proliferation, and collagen synthesis (15). Prolonged administration of corticosteroids is associated with the development of oral ulcers, which can be particularly painful and significantly detract from a patient’s quality of life. This condition is primarily attributed to immunosuppression associated with corticosteroid therapy, leading to alterations in the oral epithelium and increased susceptibility to ulceration (15).
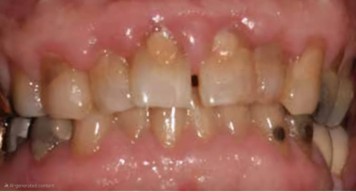
Figure 2: Cyclosporine-induced gingival enlargement (26).
Effects on Bone Stability
Corticosteroids affect the electrolyte and water balance by stimulating the reabsorption of sodium, the excretion of potassium, hydrogen ions, and calcium and decreasing the absorption of calcium (27, 28). This results in hypokalemia, hypocalcemia, hypernatremia, and alkalosis (27). In addition to increasing fracture rates and bone loss (28). Bone loss associated with prolonged corticosteroid therapy is characterized by two distinct phases. The initial phase is marked by a rapid decline in bone density, with losses ranging from 10% to 15% occurring within the first few months of treatment. This is followed by a second phase, where bone loss progresses at a more gradual rate, accounting for an annual decrease of approximately 2% to 5% thereafter (29-32). The rate of bone loss and the total amount of bone density reduction are significantly correlated with both the duration and dosage of corticosteroid administration. Specifically, when corticosteroids are prescribed at a daily dosage exceeding 7.5 mg for a duration longer than six months, there is a substantial risk of developing bone loss and, consequently, osteoporosis (27). In a study conducted by Beeraka et al., patients were administered prednisolone at an average dose of 7.04 mg over a mean duration of 10.58 months. Remarkably, there were no reported cases of corticosteroid-induced fractures among the participants. This finding is particularly significant given the well-documented concern regarding the adverse effects of corticosteroids on bone health. Furthermore, the researchers observed that the rate of bone loss in trabecular bone, predominantly found in the maxilla, is significantly more rapid than the rate of bone loss observed in cortical bone, which is primarily located in the mandible (27). Therefore, fracture is more frequent in trabecular bone (27). Deepak et al. reported a case of allergic bronchopulmonary aspergillosis that developed a nasal septal perforation due to the prolonged use of inhaled corticosteroids (33).
The World Health Organization (WHO) defined Osteoporosis as a disease characterized by decreased bone mass, bone fragility, increased susceptibility to fractures, and micro-architectural deterioration of bone tissue (34). It often occurs in women. It was reported that women who suffer from osteoporosis may experience a notable loss of periodontal attachment, with no major differences regarding gingivitis (15). Another study conducted by Ronderos et al. confirmed the association between periodontal attachment loss and bone mineral loss (15). Osteoporosis is considered one of the serious adverse effects of long-term corticosteroids. It is one of the factors that lead to low bone mineral density (BMD) (35). Bone mineral density (BMD) serves as a critical parameter in evaluating bone health and is a significant indicator of bone loss. However, it is important to note that BMD alone does not provide a comprehensive assessment of fracture risk. The evaluation of fracture risk is a multifaceted process that necessitates the consideration of additional factors. These include various clinical risk factors, as well as both the macrostructural and microstructural properties of bone (36). Bone mineral density is measured by dual-energy X-ray absorptiometry (DEXA). Cancellous bone microarchitecture is crucial to measure bone strength; however, it cannot be measured by DEXA. To overcome this issue, a trabecular bone score was developed to assess bone microarchitecture (36). Bone mineral density decreases in trabecular bone and cortical bone during corticosteroid therapy (27). During corticosteroid therapy, the jaws have a decreased BMD, which can lead to tooth loss due to the constant masticatory force (27). Moreover, a decreased BMD can cause the resorption of the bone structure and alveolar sockets supporting the teeth (35). Komerik et al. reported that individuals with low BMD have fewer teeth compared to individuals with normal MBD (35). Olgaard et al. conducted a comprehensive study indicating that long-term administration of corticosteroids leads to a notable reduction in the mineral content of bone tissue. However, their findings also revealed significant differences in the rates of bone resorption across various anatomical sites, specifically within the forearm, mandible, and lumbar spine. This variability may have important implications for understanding localised skeletal effects in corticosteroid-treated patients (37). There are important biochemical markers involved in bone metabolism, which are type I procollagen carboxy propeptide, urinary hydroxyproline serum, and alkaline phosphatase (S.ALP). By observing these biochemical markers, we can indicate when an issue arises. For instance, a notable negative correlation was observed between the activity of alkaline phosphatase enzyme and decreased bone mass. Additionally, there is a significantly low calcium serum level in patients who receive long-term corticosteroid therapy (15). This finding suggests that these patients experience calcium absorption as well.
To avoid such negative effects of prolonged use of corticosteroids, patients should follow a diet rich in calcium and vitamins, specifically vitamin D. In addition, regular measurement of mandibular and maxillary BMD and regular dental checkups are recommended to detect the adverse effects of the drug and assess the risk of osteoporosis (15).
Conclusion
Immunosuppressive agents play a vital role in the management of various diseases and in the prevention of acute and chronic rejection during organ transplantation. Nevertheless, the prolonged utilization of these agents is associated with a spectrum of adverse effects affecting systemic health and, notably, the oral cavity. These effects can impair tissue stability and bone integrity, leading to conditions such as candidiasis, periodontitis, and gingivitis, as well as negatively impacting bone metabolism and increasing the risk of osteoporosis. Current research remains insufficient in addressing strategies to mitigate the adverse consequences of long-term immunosuppressive therapy on oral health.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding.
Ethical consideration
Non applicable.
Data availability
All data is available within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.