Volume 5, Issue 11
November 2025
A Systematic Review of the Integration of Palliative Care in Dialysis Treatment
Abdulhakeem Assiri, Abdulrahman Abdullah Korairi, Ibrahim A. Ahmed, Abdulrhman Alshehri, Abdulrahman E. Altowairqi
DOI: http://dx.doi.org/10.52533/JOHS.2025.51118
Keywords: end-stage renal disease, ESRD, palliative care, dialysis, symptom management, quality of life, advance care planning
End-stage renal disease (ESRD) patients on dialysis often experience a high symptom burden, reduced quality of life, and limited engagement in advance care planning. Palliative care interventions, which focus on symptom relief and supportive care, have the potential to address these needs. However, the impact of such interventions on health outcomes, symptom management, and advanced care planning in this population remains unclear. We conducted a systematic review to evaluate the effectiveness of palliative care interventions in ESRD patients on dialysis. We searched PubMed, Embase, and Cochrane Library for studies published between 2000 and 2024. Inclusion criteria focused on studies that reported on clinical outcomes, quality of life, symptom burden, and advanced care planning. Data extraction and quality assessment followed a structured protocol, with clinical endpoints including symptom relief, quality of life improvements, and advance directive completion. A total of 11 studies were included in the review, covering various palliative care approaches such as telehealth consultations, multidisciplinary care, and symptom-specific management programs. The findings suggest that palliative care interventions can significantly improve quality of life and alleviate symptoms such as pain, fatigue, and psychological distress. Furthermore, advance care planning outcomes were enhanced, with increased documentation of patient preferences and improved patient-provider communication. However, heterogeneity in study designs and outcome measures limited the comparability of results. Palliative care interventions show promise in enhancing quality of life, symptom management, and advanced care planning for ESRD patients undergoing dialysis. Despite these positive outcomes, further research with standardized intervention protocols and long-term follow-up is essential to determine the consistent benefits of palliative care in this population.
Introduction
Chronic kidney disease (CKD) refers to various disorders affecting kidney function and structure. The 2002 guidelines marked a shift in recognizing CKD as a global public health issue that general internists should manage early. CKD is classified by severity based on glomerular filtration rate (GFR), albuminuria, and clinical diagnosis. It can be detected through routine tests, and treatments can slow progression, reduce complications, lower cardiovascular risk, and improve survival and quality of life (1). The development and progression of CKD, including end-stage renal disease, remain major contributors to diminished quality of life, premature mortality, and an increased need for palliative care to manage complex symptoms and improve patient well-being.
Globally, over 850 million people are affected by kidney disease, with 843.6 million cases attributed to CKD. From 1990 to 2016, CKD incidence and prevalence rose by 89% and 87%, respectively, with the increase exceeding 100% in countries with low and middle sociodemographic indices. CKD-related deaths doubled in three decades, moving CKD from the 18th to the 11th leading cause of death globally by 2016 (2).
The early development of dialysis by pioneers like Willem Kolff and Belding Scribner transformed kidney failure treatment, impacting its epidemiology, economics, and ethics. Despite the expansion of dialysis, especially in high-income countries, patient-centered innovation has slowed. Current costs are unsustainable, and globally, many people with kidney failure cannot access treatment, leading to millions of deaths annually. There is a pressing need for cost-effective, accessible dialysis options that improve patient outcomes (3).
The World Health Organization (WHO) defines palliative care as a method aimed at enhancing the quality of life for individuals with serious illnesses and their families. While awareness and understanding of palliative care have grown among healthcare professionals over the past decade (4).
In current practice, palliative care is typically reserved for patients whose curative treatments have been deemed ineffective. As a result, many healthcare providers equate palliative care with end-of-life care, often initiating it only when life-prolonging treatments are stopped. However, limiting palliative care to the final stages of life overlooks patients' physical and emotional needs throughout the course of their illness. Today, it is recommended that palliative care be provided alongside life-sustaining treatments from the moment a serious disease, like cancer or chronic organ dysfunction, is diagnosed, forming part of a comprehensive care plan for all patients (5).
Palliative dialysis shifts the focus from conventional disease-centered treatment to a patient-centered approach, emphasizing comfort and alignment with personal goals to enhance quality of life and alleviate symptoms in dialysis patients during their final year. This approach is recommended for those with limited life expectancy who wish to reduce the burden of treatment. Palliative dialysis is particularly suited for specific situations: (i) maintenance dialysis patients with a short life expectancy, (ii) those who develop a severe illness that significantly shortens life expectancy, (iii) patients started on dialysis due to acute kidney failure with uncertain prognosis, and (iv) maintenance dialysis patients experiencing progressive functional or cognitive decline (6).
The objective of this systematic review is to evaluate the current evidence on the integration of palliative care in dialysis treatment. It aims to assess the impact of this integration on patient outcomes, including quality of life, symptom management, decision-making processes, and healthcare utilization, while identifying challenges and best practices in delivering palliative care alongside dialysis.
Methods
Study design
This systematic review study, conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Definition of outcomes and inclusion criteria
The population included patients undergoing dialysis treatment (hemodialysis or peritoneal dialysis) for chronic kidney disease. The intervention focused on the integration of palliative care into dialysis treatment, which involved any coordination or incorporation of palliative care services into the routine care of these patients. The aim was to improve the quality of life and address symptoms rather than focusing solely on disease progression. The comparison group consisted of patients receiving standard dialysis treatment without integrated palliative care or without coordinated palliative interventions. The outcomes evaluated in the studies included quality of life, symptom management (e.g., pain, fatigue, mental health), patient and family satisfaction, end-of-life care decisions, and overall patient survival.
Search Strategy
A comprehensive search strategy will be employed to identify relevant studies on the integration of palliative care in dialysis treatment. The search will be conducted across five major databases: PubMed, Scopus, Web of Science (WOS), ScienceDirect, and the Cochrane Library for studies published between 2000 and 2024. The search terms will include a combination of keywords such as "palliative care," "end-of-life care," and "supportive care," alongside "dialysis," "renal dialysis," "kidney dialysis," "hemodialysis," and "peritoneal dialysis." Additionally, terms like "integration," "coordination," "combined," and "incorporation" will be used to focus on studies examining how palliative care is integrated into dialysis. This approach will ensure broad coverage of the relevant literature, facilitating a thorough review.
Screening and Extraction
Articles with irrelevant titles were excluded from consideration. In the subsequent phase, both the full text and abstracts of papers were meticulously reviewed to determine their compliance with the inclusion criteria. To streamline the process, titles and abstracts were organized, assessed, and scrutinized for any duplicate entries using reference management software (Endnote X8). To ensure the highest quality of selection, a dual screening approach was adopted, involving one screening for the evaluation of titles and abstracts, and another for the comprehensive examination of the entire texts. Once all relevant articles were identified, a structured extraction sheet was created to capture pertinent information aligned with our specific objectives.
Two separate researchers conducted the data extraction process independently. The gathered information included various study attributes like the author's name, publication year, country of origin, study design, sample size, duration of follow-up, and sources of funding. Additionally, details regarding participants, such as age, gender, and nationality, were also collected.
Quality Assessment
In our systematic review, we employed the Newcastle-Ottawa Scale (NOS) as a critical tool for assessing the quality of non-randomized studies included in our analysis (7). The NOS is widely recognized for its utility in evaluating the methodological quality and risk of bias in observational studies, including cohort and case-control studies. It provides a structured framework for evaluating key aspects of study design, such as the selection of study groups, comparability, and ascertainment of outcomes. Additionally, for randomized controlled trials (RCTs), we used the Cochrane Risk of Bias tool to assess the quality and potential biases (8). This tool allows for a thorough examination of factors such as randomization, blinding, and incomplete outcome data. By using both the NOS and the Cochrane Risk of Bias tool, we systematically appraised the included studies, ensuring that only high-quality evidence contributed to our analysis, thereby enhancing the robustness and reliability of our findings.
Results
Search results
We executed the search methodologies outlined previously, resulting in the identification of a total of 272 citations, subsequently reduced to 194 following the removal of duplicates. Upon screening titles and abstracts, only 35 citations met the eligibility criteria for further consideration. Through full-text screening, this number was further refined to 11 articles (9-19) aligning with our inclusion and exclusion criteria. Figure 1 provides an in-depth depiction of the search strategy and screening process.
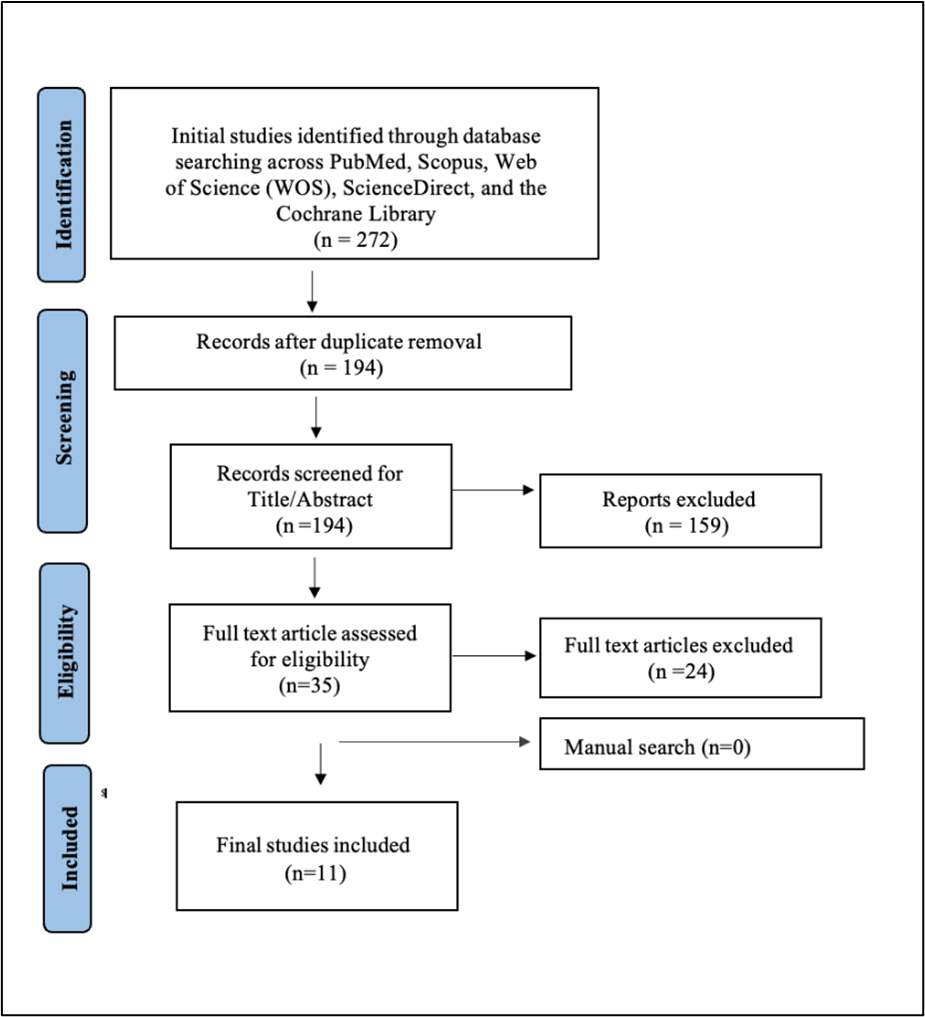
Figure 1: PRISMA flowchart
Results of quality assessment
Our systematic review, guided by the NOS, highlights the varying quality of non-randomized studies assessing the integration of palliative care in dialysis treatment. Several studies, such as those by Tamura et al. (2022) (10), Evans et al. (2023) (13), and Siriwardana et al. (2020) (15), scored 9 stars, demonstrating robust methodologies across selection, comparability, and outcome assessment, providing strong evidence supporting the integration of palliative care in dialysis to improve patient outcomes. However, some studies, like those by Ducharlet et al. (2021) (12) and Pungchompoo et al. (2021) (14), received lower scores due to limited comparability or incomplete follow-up, indicating the need for more rigorous designs and consistent reporting.
Overall, while high-scoring studies offered reliable data on the benefits of integrating palliative care in dialysis treatment, the review underscores the importance of continued high-quality research to further validate these findings. This will ensure that palliative care is effectively incorporated into dialysis treatment to enhance patient quality of life, symptom management, and end-of-life care decisions, ultimately improving overall patient well-being (Table 1).
|
Table 1. Summary of the results of bias assessment of the included studies using the modified Newcastle-Ottawa scale (NOS) for observational studies |
||||||||||
|
Author |
Selection |
Comparability |
Outcome |
Total score |
||||||
|
Representativeness of the exposed cohort |
Selection of a nonexposed cohort |
Ascertainment Of the Exposure |
Outcome does not present at the start of the study |
Subjects in different outcome groups are comparable |
Assessment of outcome |
Length of follow-up |
Adequacy of follow-up |
|||
|
Lipstiz et al., 2024 (9) |
* |
* |
* |
* |
**** |
|||||
|
Tamura et al., 2022 (10) |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Cheung et al., 2021 (11) |
* |
* |
* |
* |
* |
* |
* |
******* |
||
|
Ducharlet et al., 2021 (12) |
* |
* |
* |
* |
* |
***** |
||||
|
Evans et al., 2021 (13) |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Pungchompoo et al., 2021 (14) |
* |
* |
* |
** |
* |
****** |
||||
|
Siriwardana et al., 2020 (15) |
* |
* |
* |
* |
** |
* |
* |
* |
********* |
|
|
Hing et al., 2016 (16) |
* |
* |
* |
* |
** |
* |
******* |
|||
|
Redahan et al., 2013 (17) |
* |
* |
* |
* |
** |
* |
******* |
|||
|
Cohen et al., 2000 (18) |
* |
* |
* |
* |
* |
* |
****** |
|||
The Cochrane Risk of Bias assessment for Li Juan et al. (2014) revealed a low risk of bias in random sequence generation and incomplete outcome data (4). However, concerns arose regarding allocation concealment, which was unclear, and high risks associated with blinding of participants and outcome assessment. These limitations suggest caution in interpreting the study's findings, as potential biases may have influenced the results (Table 2).
|
Table 2. Cochrane Risk of Bias tool for RCT studies |
|||||||
|
Study |
Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective reporting |
Other bias |
|
Li Juan et al., 2014 (19) |
Low |
Unclear |
High |
High |
Low |
Low |
High |
Characteristics of included studies
A total of 11 studies, published between 2000 and 2024, were included in the review. The baseline characteristics of the included studies reveal a diverse demographic profile across various designs and countries, primarily focusing on elderly male patients undergoing dialysis treatment. The total sample size across all studies was 3,547, with males comprising 55.78% of the participants. The studies were conducted in multiple countries, including the USA, Canada, Australia, New Zealand, Thailand, Malaysia, Ireland, and China. Notably, 10 of the studies were non-randomized, while only one study was randomized (4). These findings highlight the predominance of older male populations, indicating a need for tailored palliative care approaches to address their specific needs (Table 3).
|
Table 3. Baseline characteristics of included studies |
|||||
|
Study ID |
Design |
Country |
Sample size |
Male, n (%) |
Age range or mean (SD) |
|
Lipstiz et al., 2024 (9) |
Cohort |
USA |
48 “10 providers, 20 nurses, and 18 caregivers”. |
NR |
NR |
|
Tamura et al., 2022 (10) |
Cohort |
USA |
273-Preimplementations 203-Postimplementations |
158(58) 12 (60) |
Pre: 71.96 (10.29) Post: 74.41 (10.19) |
|
Cheung et al., 2021 (11) |
Single-arm pilot clinical trial |
USA |
36 |
22 (61) |
70.8 (10.9) |
|
Ducharlet et al., 2021 (12) |
Cross-sectional |
Australia and New Zealand |
382 “Doctor: n = 123 Nurse: n = 259” |
88 (23.0) |
NR |
|
Evans et al., 2021 (13) |
Survey |
Canada |
1925 |
1155(60.0) |
NR |
|
Pungchompoo et al., 2021 (14) |
Descriptive |
Thailand |
100 |
51 (51.0) |
68.32 (7.61) |
|
Siriwardana et al., 2020 (15) |
Prospective |
Australia |
127 |
79 (62.0) |
73.7 (11.02) |
|
Hing et al., 2016 (16) |
Cross-sectional |
Malaysia |
56 |
32 (57.1) |
59.5 (10.9) |
|
Redahan et al., 2013 (17) |
Retrospective chart review |
Ireland |
131 “Palliative care involvement (n = 48) No palliative care involvement (n = 83)”. |
88 (67.2) |
63.2 (15.1) |
|
Cohen et al., 2000 (18) |
Prospective, observational cohort |
USA and Canada |
131 undergoing dialysis 79 (60%) were prospectively studied until their deaths |
NR |
70 (1.2) |
|
Li Juan et al., 2014 (11) |
RCT |
China |
Total=135 “Case: n = 69 Control: n = 66” |
79(58.5) |
56.3 (12.4) |
NR, not reported; RCT, Randomized Controlled Trial
Outcomes within the included studies
The studies assessed various aspects of patient quality of life (QOL), symptom management, patient and family satisfaction, end-of-life care decisions, and overall patient survival in accordance with types of palliative care programs or systems.
Type of palliative care program or system
In Lipsitz et al. (2024), evaluated hospital-based, consultative palliative care program with pediatric nephrology was evaluated for children with end-stage kidney disease (ESKD) receiving dialysis (9). Tamura et al. (2022) reported that serious illness screening, goals of care discussions, and palliative dialysis care pathways increased from two to five centers (10). Cheung et al. (2021) found a telepalliative care consultation, four specialty palliative care clinicians, receiving dialysis (11). Ducharlet et al. (2021) evaluated renal supportive care (RSC), Specialist palliative care services (SPC) (12) Evans et al. (2021) found coordinated care delivery for patients with advanced Chronic kidney disease (CKD) (13). Pungchompoo et al. (2021) found a home telehealth model in end-of-life care for OPLH (14). Siriwardana et al. (2020) found RSC (15). Hing et al. (2016). Advance care planning decisions among end-stage renal disease (16). Redahan et al. (2013) reported that SPC (36.7%) in patients with ESKD (17). Cohen et al. (2000) noted the terminal course of a group of patients who died after dialysis discontinuation (18). Li Juan et al. (2014) post-discharge nurse-led telephone support care (19).
The findings collectively emphasize the critical role of palliative care in enhancing the quality of life for dialysis patients. For example, in Lipsitz et al. (2024), 80% of providers and all nurses agreed that palliative care benefits dialysis-dependent pediatric patients, with 22% of caregivers finding palliative care helpful (9). Ducharlet et al. (2021) noted 97% of patients saw palliative care improvement, and 89% of patients saw RSC improvement (12). Also, Li Juan et al. (2014) found significant improvements in support care (19).
Many studies highlighted the importance of integrating palliative care services into routine care to better address symptom management and improve patient and caregiver satisfaction. For instance, Lipsitz et al. (2024) reported that uncomfortable physical symptoms and 100% found to be helpful for patient and family satisfaction (9). While Cheung et al. (2021) found that 81% of patients acceptable for treatment is relevance, with a mean quality of life score of 7.3 on a 0–10 scale (11). Siriwardana et al. (2020) demonstrated significant improvements in physical and emotional symptoms over three visits (15). Hing et al. (2016) found that 69.6% of participants wanted CPR in a cardiorespiratory collapse outside the dialysis center, with an increased awareness of advance care planning (16). Finally, Li Juan et al. (2014) found significant improvements in patient satisfaction and well-being (19).
Many studies have found an end-of-life care decisions. For example, Tamura et al. (2022) reported a notable 34.5 percentage point increase in advance care planning documentation post-implementation of a learning collaborative for hemodialysis centers during the COVID-19 pandemic (10). Redahan et al. (2013) reported that 36.7% of patients were referred to specialist palliative care, often at a late stage, highlighting the need for better integration of palliative care in end-of-life situations (17). Cohen et al. (2000) (18) emphasized that integrating palliative care into dialysis programs improves end-of-life care, noting common symptoms experienced by patients (18). Overall survival was measured in Cohen et al. (2000), mean time was 8.2 days (18) (Table 4).
Overall, these studies indicate a strong need for more comprehensive palliative care approaches in the dialysis setting, especially considering the high prevalence of complex symptoms and the importance of advance care planning. Integrating these services could lead to improved patient outcomes and satisfaction in the treatment of chronic kidney disease.
|
Table 4. Main outcome of Included studies |
|||||||
|
Study ID |
Type of palliative care program/system |
QOL |
Symptom management |
Patient and family satisfaction |
End-of-life care decisions |
Overall patient survival |
Conclusion |
|
Lipstiz et al., 2024 (9) |
The study evaluated a hospital-based, consultative palliative care program, pediatric nephrology for children with ESKD receiving dialysis. |
80% of providers and all nurses agreed that palliative care benefits dialysis-dependent pediatric patients. 22% of caregivers had children who received palliative care. |
Uncomfortable physical symptoms. |
100% found it helpful |
90% of providers and 100% nurses wanted more palliative care education. |
NR |
The data highlight the need for more palliative care education and greater involvement of palliative care in pediatric nephrology. |
|
Tamura et al., 2022 (10) |
Serious illness screening, goals of care discussions, and palliative dialysis care pathway increased from two to five centers. |
NR |
NR |
NR |
The adjusted probability of complete advance care planning documentation among patients increased by 34.5 percentage points from pre- to post-implementation. Among the remaining nine centers, 20% (273 of 1395) of patients were identified as seriously ill pre-implementation, while 16% (203 of 1254) were identified post-implementation. |
NR |
A learning collaborative for hemodialysis centers during the COVID-19 pandemic led to increased adoption of serious illness screening, goals of care discussions, and better documentation of advance care planning for seriously ill patients. |
|
Cheung et al., 2021 (11) |
A telepalliative care consultation, four specialty palliative care clinicians, receiving dialysis. |
80% of patients found the teleconsult as better than an in-person visit. 41% of patients preferred teleconsult |
Physical symptoms: 2 (1,3), Emotional symptoms: 1 (1.2) |
Acceptable, 81% of patients found that the treatment is relevance 58% of patients learned new information 27% of patients found that the appointment altered their perspective on dialysis. Mean score : 7.3 ± 1.5 on a 0–10 scale, with 10 being the highest. |
Change in ilness:28% yes, 38% unsure, and 34% no; to continued discussion:56% yes, 34% may be; 84% relevant. |
NR |
The teleconsultation experience was well-received, with a significant majority finding it as beneficial as in-person visits, while a substantial portion also gained new insights or experienced a shift in how they view their treatment. |
|
Ducharlet et al., 2021 (12) |
RSC, Specialist palliative care services |
Palliative care improved: 97%; RSC improved: 89% |
relief 86% |
more acceptable than palliative care (80%) with preferential RSC referral for (86% vs 69%) and complex treatment decisions (82% vs 58%). |
conversations delayed: (72%); lack of agreed treatment goals by the patient, family or treatment team (86%), late or rushed treatment: 85% |
NR |
It presents opportunities to enhance RSC by aligning it with clinicians' priorities to improve patient care. |
|
Evans et al., 2021 (13) |
Coordinated care delivery for patients with advanced CKD |
NR |
NR |
37.4% well-coordinated across different settings; 56.2% inter-disciplinary discussions; 76.1% easy access to medical history |
NR |
NR |
Improving communication among healthcare professionals and increasing awareness of home- and community-based services can enhance patient perceptions of coordinated care. |
|
Pungchompoo et al., 2021 (14) |
A home telehealth model into end-of-life care for OPLH |
Physical: 45.56; general: 49.78 |
Shortness of breath (25%), rash (30%), pain (31%), swelling (31%), nausea and vomiting (15%, anorexia (30%), depression (8%);81% no readmission |
65% always had time to discuss their problems with their doctors;79% satisfied with involvement for decisions about their care and self-treatment;12% wanted more involved in these decisions. |
NR |
NR |
Participants highlighted telehealth as a crucial aspect of their care, including VDO visits, telephone counseling, and web-based education/monitoring. |
|
Siriwardana et al., 2020 (15) |
RSC |
NR |
Difficulty in sleeping (35%), pain (31%), lack of energy (31%), poor morbidity (24%), itch (22%). Significant improvement in physical and emotional symptom scores over three visits (mean change −3.8, P < 0.001). |
Symptom improvements were sustained at final visit (median 13 months), without changes in dialysis delivery. |
NR |
NR |
RSC intervention focusing on symptom control and patient-centered care significantly reduces total and individual symptom burdens in dialysis patients, supporting its role as an effective management adjunct. |
|
Hing et al., 2016 (16) |
Advance care planning decisions among end-stage renal disease |
NR |
69.6% (n = 39) want CPR in a cardiorespiratory collapse outside the dialysis center. 82.1% (n = 46) want resuscitation during dialysis (significant difference, P = 0.001). |
NR |
64.3% (n = 36) believe early preparation of ACP is important. 67.9% (n = 38) expressed plans to prepare ACP post-survey; no significant change after education (P = 0.146). Chances of survival in the event of the cardiorespiratory collapse were <10%, almost half of the participants, n = 27 (48.2%) chose not for CPR |
NR |
Following exposure to the educational brochure, participants exhibited an upward trend in the importance of advance care planning (ACP) and CPR decisions, though not statistically significant. Notably, 75% had never heard of ACP, and only 3.6% had prepared a written advance directive. |
|
Redahan et al., 2013 (17) |
SPC (36.7%) in patients with ESKD |
NR |
NR |
NR |
Dialysis was withdrawn in 50 cases; DNR order was 51.9%. 79.4% died in an acute hospital, 14.5% at home, 2.3% in hospice, and 3.8% unknown |
NR |
SPC was involved in care for about a third of patients, often at a late stage. With short timeframes after dialysis withdrawal, better integration of palliative care and nephrology is needed to improve end-of-life care for ESKD patients. |
|
Cohen et al., 2000 (18) |
Terminal course of a group of patients who died after dialysis discontinuation |
NR |
Pain medication (87%); Oxygen (22%); treatment was effective for 93% |
NR |
Inpatients hospice: 2% 60% formal advance directive; Patients received terminal care either in the hospital (61%), nursing home (24%), inpatient hospice (2%), or at home (13%); Families and/or staff were present at the time of death in 71%. |
Mean time: 8.2 days |
Integrating palliative care into dialysis programs improves end-of-life care, especially for those stopping treatment. Despite common symptoms like pain and agitation, terminal care was satisfactory for most patients |
|
Li Juan et al., 2014 (19) |
Post-discharge nurse-led telephone support care |
Improved in KDQOL-SF(significant symptoms,fatigue, satisfaction) |
better sleep, pain control and energy fatigue. |
Significant in intervention group. |
NR |
Nurse-led telephone support after discharge for peritoneal dialysis patients effectively improves their well-being during the transition from hospital to home in mainland China. |
|
RSC, renal supportive care; NR, not reported; QOL, quality of life; SPC, specialist palliative care; ACP, advance care planning; VDO, video; KDQOL-SF, kidney disease quality of life-short form; ESKD, end-stage kidney disease; OPLH: older persons living with hemodialysis DNR: do not resuscitate; CKD: Chronic kidney disease.
Discussion
Palliative care is increasingly recognized as vital in managing chronic conditions like end-stage renal disease (ESRD), particularly for patients undergoing dialysis. Despite its potential to improve quality of life by addressing pain, symptom burden, and psychosocial needs, the integration of palliative care into dialysis settings remains inconsistent and often insufficient. Understanding these aspects can help improve care for dialysis patients, offering them more comprehensive and compassionate treatment options. This review highlights the pivotal role of palliative care in enhancing the quality of life for patients with renal disease undergoing dialysis. Our findings reveal that while palliative care significantly improves symptom management, emotional support, and patient satisfaction, systemic barriers continue to obstruct its widespread adoption.
Summary of Findings
The reviewed evidence supports the integration of palliative care into nephrology as a means of improving quality of life, symptom management, patient satisfaction, and end-of-life care for patients with advanced kidney disease. Whether delivered through hospital-based programs, renal supportive care clinics, or telehealth platforms, palliative care consistently enhances patient-centered outcomes. Nonetheless, gaps persist, particularly in early referral, provider education, and advanced care planning awareness. Future strategies should focus on expanding access to renal supportive care, incorporating telepalliative approaches, and training healthcare professionals to deliver comprehensive, timely, and compassionate care tailored to the complex needs of ESKD patients.
Telepalliative Care: A Promising Approach
Telepalliative care has emerged as a highly promising model for improving access, continuity, and quality of palliative services among patients with advanced kidney disease. Studies such as Cheung et al. (2021) (11) and Pungchompoo et al. (2021) (14) demonstrate that remote consultations and home-based telehealth interventions can deliver care outcomes comparable to, or even better than, traditional in-person visits. Cheung et al. (11) reported that 80% of patients found teleconsultations superior to face-to-face meetings, 81% considered them relevant to their needs, and 58% gained new insights into their treatment, achieving a high mean satisfaction score of 7.3 ± 1.5. Likewise, Pungchompoo et al. (14) found that telehealth-based end-of-life programs achieved mean quality of life scores of 45.56 (physical) and 49.78 (general), with 81% of participants avoiding hospital readmission and 79% expressing satisfaction with decision-making involvement. These findings illustrate how telepalliative care bridges geographical and logistical gaps, enhances communication between patients and providers, and supports timely symptom management and shared decision-making. By combining virtual consultations, telephone counseling, and web-based education, telepalliative care offers a flexible, cost-effective, and patient-centered approach that aligns well with the growing demand for accessible, holistic renal supportive care. According to Davison (2010), telehealth can mitigate barriers to access, ensuring that vulnerable populations receive timely palliative care interventions (20). In recent times, telepalliative care, which leverages telehealth technologies to deliver palliative services remotely, has shown significant promise in addressing these challenges.
Cheung et al. (2022) further supported these findings by examining the content of telepalliative care consultations with dialysis patients, analyzing video recordings from a pilot program conducted at five facilities (21). Of the 39 recruited patients, 34 completed consultations with palliative care clinicians using mounted screens and iPads. Each conversation lasted an average of 42 minutes and addressed key themes, including sources of strength (91%), critical abilities (88%), illness understanding (85%), and fears (85%). However, emotional expression was less common, occurring in only 21% of discussions, with silence noted in 56%. This qualitative analysis demonstrated that telepalliative care effectively covered essential domains outlined by the Serious Illness Conversation Guide (SICG), even in the open setting of a dialysis unit. These findings underscore the value of telepalliative care in providing holistic support that meets both informational and emotional needs, laying the groundwork for future remote palliative care models.
Further studies, such as those by Morgenstern-Kaplan (2021), reinforce these findings by demonstrating that telehealth interventions can enhance patient satisfaction in a more generalized setting (22). Additionally, they facilitate crucial discussions about care goals. Vahlkamp et al. (2024) stated that telepalliative services are increasingly recognized as viable for engaging patients with complex chronic conditions, such as advanced CKD, in goals of care (GOC) conversations (23). They conducted a mixed-methods pilot study involving U.S. Veterans found telehealth feasible for facilitating GOC discussions, as older Veterans with advanced CKD engaged effectively in these conversations across visit modalities. Despite barriers, including limited non-palliative care provider involvement and uncertainty about illness trajectory, telehealth consultations fostered reassurance and aligned life-sustaining treatment choices with patient preferences. These findings support telehealth as a promising model for GOC in CKD patients, enhancing accessibility and the potential for consistent patient-centered care.
The advantages of telepalliative care extend beyond geographic considerations; they also address temporal barriers. Traditional palliative care models often require in-person visits, which can be challenging to coordinate due to patients’ fluctuating health conditions. Telepalliative care allows for more flexible scheduling and can provide timely interventions in response to emerging needs (24). For example, a patient experiencing increased pain or distress can quickly connect with a palliative care specialist without waiting for the next in-person appointment, thereby potentially preventing crises that might lead to emergency interventions. Moreover, telepalliative care has the potential to enhance interdisciplinary collaboration among healthcare providers. It allows for more frequent consultations between palliative care teams and dialysis providers, fostering a team-based approach to patient management (25). This can lead to improved coordination of care, as telehealth platforms facilitate the sharing of information and resources among providers, ultimately benefiting patient outcomes.
However, while the findings thus far are promising, it is essential to consider the barriers to implementing telepalliative care. A systematic review by Chen et al. (2022) suggested that while tele-palliative care shows potential for enhancing physical health and supporting the psychological well-being of both patients and caregivers, evidence remains limited regarding its overall effectiveness in practice (26). Technological challenges, such as varying levels of digital literacy among patients and limited internet access in rural areas, can hinder the effectiveness of telehealth solutions. Additionally, ensuring that telepalliative care is reimbursed appropriately by insurance providers remains a critical consideration for widespread adoption (27). Addressing these challenges will require ongoing efforts from healthcare systems, policymakers, and technology developers to ensure equitable access to telepalliative care services.
Early Integration of Palliative Care
Early integration of palliative care into the management of chronic and end-stage kidney disease is essential to improving patient outcomes, quality of life, and decision-making throughout the disease trajectory. Evidence from multiple studies highlights that timely involvement of palliative services allows for better symptom control, enhanced communication, and more meaningful advance care planning (ACP). For instance, Tamura et al. (2022) (10) found that introducing a structured palliative dialysis care pathway increased ACP documentation by 34.5%, while Hing et al. (2016) (16) reported that 82% of patients valued early discussions about care preferences, despite only 3.6% having written directives. However, Redahan et al. (2013) (17) revealed that most patients (79.4%) still died in acute hospital settings, reflecting late palliative referral and limited continuity of care. Early integration can help shift this pattern by promoting proactive symptom management, emotional support, and alignment of treatment goals with patient values. It also facilitates interdisciplinary coordination, as demonstrated by Evans et al. (2021) (13), who found improved care continuity through better communication among healthcare teams. Overall, incorporating palliative care from the early stages of kidney disease rather than reserving it for the terminal phase ensures that patients receive holistic, person-centered support that enhances dignity, comfort, and autonomy throughout their care journey. Wang et al. (2022) support the urgency for early palliative interventions by documenting a high symptom burden among patients with ESRD on maintenance hemodialysis, with older patients experiencing even greater needs (28). The study concluded that both younger and older patients face substantial physical discomforts like dry mouth, itching, and skin issues, which contribute to a significant decrease in health-related quality of life (HRQOL). Integrating palliative care early on could address these pervasive symptoms while offering a holistic approach to patients’ physical, psychological, and spiritual needs, thus enhancing their overall quality of life and reducing symptom severity from the outset.
Shifting the perception of palliative care to encompass support throughout the entire illness journey is essential for expanding its reach and impact within nephrology settings. When palliative care is viewed as a complementary approach to curative treatments rather than an alternative, it opens the door to a more holistic model of patient-centered care. This perspective aligns with the WHO's definition of palliative care, which emphasizes the importance of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial, and spiritual (29). Along these lines, Tamura et al. (2018) propose reorienting CKD care to focus on “early goals of care conversations” rather than solely preparing patients for dialysis, offering symptom management and decision support across diverse patient goals, including those seeking conservative care without dialysis (27).
Furthermore, integrating palliative care early in the treatment process can help patients make informed, proactive decisions about their treatment. Cohen et al. (2018) highlight that while palliative care has been widely adopted in oncology, it is less frequently offered to nephrology patients, often only considered after dialysis begins and patients are already questioning its benefits (30). Their project showed that by offering palliative care at earlier stages of CKD, patients had the opportunity to set goals and consider conservative treatment options before experiencing end-stage symptoms. Importantly, the study found that this approach helped reduce the perception of palliative care as a 'death sentence,' supporting it as a component of standard care that empowers patients to take charge of their goals in a non-crisis state. Additionally, earlier referrals to palliative care allow patients with life-limiting illnesses like CKD to receive timely support and better manage their quality of life by improving the collaboration between nephrologists and palliative care specialists. This collaborative approach can foster a multidisciplinary team environment where both symptom management and psychosocial support are prioritized. In practice, this means that nephrologists can focus on the technical aspects of dialysis while palliative care professionals address the holistic needs of the patient, including emotional support, family dynamics, and spiritual concerns.
Despite these benefits, several barriers to early integration of palliative care persist. Many healthcare providers may lack the training or resources necessary to implement palliative care effectively. Moreover, systemic challenges, such as fragmented care models and insufficient reimbursement for palliative services, can impede efforts to incorporate palliative care into the standard treatment paradigm for ESRD (27).
To overcome these challenges, healthcare systems must promote educational initiatives that inform both providers and patients about the role and benefits of palliative care. This can include training for nephrologists on how to initiate conversations about palliative care and integrate it into treatment plans. In addition, raising awareness among patients about the availability of palliative care as part of their treatment options can empower them to advocate for this support early in their care journey.
Organizational and Cultural Barriers
Integrating palliative care into dialysis settings is often complicated by a myriad of organizational complexities that can obstruct timely and effective interventions.
Despite the proven benefits of palliative care in kidney disease management, its implementation remains hindered by significant organizational and cultural barriers. Healthcare systems often lack clear protocols, trained staff, and dedicated resources for integrating palliative care within nephrology services. Studies such as Hing et al. (2016) (16) and Redahan et al. (2013) (17) reveal that late referrals and hospital-centered end-of-life care, where over 60–80% of patients die in acute settings, reflect institutional inertia and inadequate coordination between renal and palliative teams. Organizationally, limited workforce capacity, absence of interdisciplinary collaboration, and insufficient education about palliative principles among nephrologists contribute to delayed or fragmented care. Culturally, both clinicians and patients may perceive palliative care as synonymous with end-of-life or “giving up,” creating resistance to early discussions about advance care planning or symptom management. For instance, despite 82% of patients valuing early conversations, only 3.6% had written advance directives (Hing et al., 2016) (16), highlighting the stigma and discomfort surrounding death-related dialogue. Additionally, family expectations, societal norms favoring life-prolonging interventions, and lack of awareness about the benefits of holistic care further impede acceptance. Overcoming these barriers requires fostering a cultural shift toward patient-centered care, enhancing professional training, and embedding palliative principles into routine nephrology practice to normalize compassionate, goal-aligned decision-making.
In Swedish nephrology settings, for example, both renal nurses and physicians have articulated that palliative care is typically associated with the cessation of dialysis treatment, which creates a significant barrier to earlier palliative interventions (31). This misconception can lead to delays in necessary palliative care discussions, ultimately impacting patients' quality of life. Such attitudes toward palliative care can be entrenched in the healthcare culture, where life-sustaining treatments are prioritized, often at the expense of holistic patient care.
To address these barriers, systemic organizational change is essential. This includes the implementation of multi-professional training programs that enhance the skills of healthcare providers in delivering palliative care and recognizing its importance early in the treatment trajectory. Such training should emphasize the role of palliative care in managing symptoms, improving quality of life, and fostering effective communication with patients and families about their values and preferences.
Furthermore, developing standardized protocols and policies that promote regular palliative assessments within nephrology settings can help ensure that palliative care is not an afterthought but rather an integral part of the patient’s care plan. These assessments can facilitate timely interventions and better coordination among multidisciplinary teams, ultimately enhancing continuity of care for dialysis patients. Clearer definitions of roles among team members can also support this integration, allowing healthcare providers to understand their responsibilities in the palliative care continuum and ensuring that patients receive comprehensive support. For instance, Davison (2010) recommends that, owing to the complex nature and scale of renal care, the implementation of identification strategies for high palliative care needs must be simple and systematically integrated (20). Assessments like the Symptom Questionnaire (SQ), modified Edmonton Symptom Assessment System (mESAS), and modified Karnofsky Performance Status Scale should be incorporated into routine clinical practice, enabling healthcare teams to recognize patients with high mortality risks. Moreover, he highlights that predicted survival probabilities can serve as a tool for identifying patients needing supportive care without the necessity for detailed documentation in medical records, thus maintaining appropriateness in communication. The integration of these assessment tools can improve healthcare teams' accuracy in prognostication, ultimately enhancing care delivery during patients' final months of life.
Further, cultural preferences and socioeconomic factors significantly influence patients' receptiveness to palliative care. For instance, research indicates that Latino patients with advanced illness often favor family-centered care models and home-based discussions for advance care planning (32).These preferences present unique challenges in standard dialysis settings, where care may be more clinic-focused and less adaptable to familial involvement. Understanding these cultural nuances is critical for healthcare providers aiming to offer sensitive and effective palliative care.
Incorporating cultural considerations into care planning can enhance the cultural sensitivity of palliative care approaches. This can involve actively engaging families in discussions about treatment options and end-of-life care, ensuring that their values and preferences are respected. However, as Lazenby et al. (2016) highlight, discussions surrounding end-of-life care are often avoided in dialysis units, where conversations about death are seen as burdensome and culturally taboo (20). In these settings, the focus tends to remain on sustaining life rather than acknowledging when a patient may be nearing the end of their natural life. To counteract this, culturally tailored educational resources can help bridge gaps in understanding and acceptance of palliative care among diverse patient populations. Addressing socioeconomic factors, such as access to transportation and financial constraints, is also vital for ensuring equitable access to palliative care services. By recognizing and addressing these barriers, healthcare systems can work toward reducing disparities in access to quality palliative care, ultimately improving health outcomes for all patients, particularly those from marginalized communities.
Clinical Implications and Recommendations
The findings across studies highlight critical clinical implications for improving palliative care delivery in patients with advanced kidney disease. Integrating palliative care early in the disease trajectory enhances quality of life, symptom control, and patient satisfaction, as evidenced by improvements in physical and emotional well-being (Siriwardana et al., 2020 (15); Li Juan et al., 2014). Telepalliative and home-based models, such as those implemented by Cheung et al. (2021) (11) and Pungchompoo et al. (2021) (14), demonstrate that remote consultations can maintain or even improve patient–provider communication, reduce symptom burden, and prevent hospital readmissions (reported at 81% no readmission). Clinicians should prioritize routine symptom assessment, structured advanced care planning, and the use of multidisciplinary teams including nurses, social workers, and spiritual counselors, to address complex physical, emotional, and social needs. Moreover, education and training programs for nephrology staff are essential; for instance, 90% of providers and 100% of nurses in Lipstiz et al. (2024) expressed the need for more palliative care education. Institutions should develop standardized care pathways that incorporate palliative consultations for high-risk or dialysis-dependent patients, ensuring timely discussions on treatment goals and end-of-life preferences. Finally, leveraging technology for telehealth and decision support systems can improve accessibility, continuity of care, and documentation of patient wishes, ultimately leading to more compassionate and person-centered management of chronic kidney disease.
Study Strengths and Limitations
This review used a systematic and comprehensive approach, ensuring broad coverage of palliative care in dialysis settings across diverse contexts. Its focus on practical, real-world interventions like telepalliative and early integration models enhances clinical relevance. However, limitations include small sample sizes, single-center studies, and reliance on self-reported data, which may introduce bias. The scarcity of high-quality RCTs also limits the strength and generalizability of the conclusions.
Overall, the quality of evidence was moderate. Studies by Tamura et al. (2022) (10) and Siriwardana et al. (2020) (15) further emphasize the need for high-quality, randomized trials to evaluate the long-term effects of integrated palliative approaches (10, 15). Most studies were concentrated in specific regions, limiting the generalizability of findings to low-resource or rural settings. The focus on ESRD patients on dialysis also restricts applicability to other chronic conditions. While telepalliative care appears promising, variations in technology access and patient familiarity affect its consistency and outcomes. Overall, these limitations highlight the need for broader, more inclusive research to strengthen evidence and ensure equitable access to palliative care across diverse populations.
Conclusion
Integrating palliative care into dialysis for ESRD patients holds substantial promise for enhancing patient-centered care and alleviating symptom burden. However, achieving widespread implementation necessitates overcoming organizational, cultural, and logistical barriers. Future research should continue to explore innovative approaches, such as telepalliative care and culturally tailored interventions, to broaden access and acceptance of palliative care among diverse patient populations. Ultimately, a proactive and inclusive approach to palliative care in dialysis could transform chronic disease management, providing patients with holistic, compassionate, and effective support throughout their care journey.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding.
Ethical consideration
Non applicable.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.