Volume 5, Issue 11
November 2025
Impact of Chronic Inflammatory States on Aneurysm Wall Stability and Rupture Risk
Sami Abdullah Alanezi, Faisal Abdullah Alghamdi, Fahad Hussein Almalki, Tharaa Younis Takrouni, Ahmed Abdulhamid Alanazi, Ahlam Hamed Al Fallaj
DOI: http://dx.doi.org/10.52533/JOHS.2025.51105
Keywords: chronic, inflammation, aneurysm, aneurysm wall stability, rupture risk
The frequency of cardiovascular diseases, such as aneurysms, continues to play a significant role in the current high death rates that are observed, even though life expectancy has increased in recent decades. In the event of an aneurysm rupture in the cerebral circulation or the abdominal aorta, the patient may experience sudden death or severe impairment. Morphological criteria like aneurysm size and growth rate have guided rupture risk assessment. Structure-only measures are limited since clinical findings show that small aneurysms can rupture while larger aneurysms stay stable. According to recent research, chronic inflammation is considered one of the most important factors that determine the stability of an aneurysm. The progressive breakdown of the wall is caused by the entry of inflammatory cells, the release of cytokines, and the activation of proteolytic enzymes. Apoptosis of vascular smooth muscle cells is induced by these mechanisms, which additionally promote neovascularization within the aneurysm wall. These mechanisms collectively enhance wall rupture risk and degradation. Consequently, hemodynamic or structural forces cannot explain aneurysm formation. However, it must be acknowledged that biological and mechanical variables interact complexly to trigger it. The intriguing techniques, such as positron emission tomography (PET) and vascular wall magnetic resonance imaging, can supplement current surveillance strategies, but study heterogeneity suggests standardization and validation. This narrative review explains chronic inflammation's effects on aneurysm wall biology using clinical, imaging, and experimental evidence. It contrasts abdominal aortic and cerebral aneurysms and explores therapeutic implications. These include risk classification models using inflammatory signs and anti-inflammatory drugs to reduce rupture risk.
Introduction
Aneurysms constitute a considerable public health issue, defined by localized expansions of artery walls that increase the risk of severe rupture in affected patients. It is important to note that the most clinically pertinent conditions are intracranial and abdominal aortic aneurysms (AAA) (1). Cerebral aneurysms can rupture and cause a fatal subarachnoid hemorrhage in 3–5% of the population, with a significantly elevated risk of morbidity and death in patients > 65 years of age (2, 3). Moreover, there is the possibility of aneurysms developing in the thoracic, visceral, and peripheral arteries, each of which has a unique set of clinical concerns (4). The diagnostic accuracy has improved due to early detection with Computed Tomography(CT), Magnetic Resonance Imaging, or ultrasound; however, predicting rupture remains a clinical challenge (5). Despite surgical and endovascular procedures, aneurysm size and its progression rate generally determine care, which fails to account for the complex nature (6).
Addressing rupture risk requires understanding aneurysm wall stability. Subsequently, larger aneurysms were considered more prone to rupture and result in catastrophic failure (7). However, clinical findings demonstrate that aneurysms of a modest size have the potential to burst, whereas those of a larger size can persist for a number of years. This mismatch shows the difficulties of using size-based criteria for clinical decision-making. A complicated interaction of biomechanical pressures, extracellular matrix integrity, smooth muscle cell activity, and hemodynamic stressors determines wall stability (8). The aneurysm wall contains biochemical and molecular components that affect the structural integrity of the wall (9). Computational modeling and improved imaging have shown rupture risk factors such as wall stress distribution, intraluminal thrombus (ILT) burden, and regional wall thickness (10).
There is growing evidence that chronic inflammatory processes play a significant role in the pathogenesis of aneurysms. These processes promote wall degradation and affect the homeostasis of the blood vessels (11). Histopathological investigations show that macrophages, lymphocytes, neutrophils, and other cells line aneurysm walls, and matrix metalloproteinases (MMPs) and elastases that are released from interleukin-6 (IL-6) and Tumor necrosis factor alpha degrade collagen and elastin. Consequently, the artery wall tensile strength and flexibility decline, leading to an increase in the rupture risk (12). Chronic inflammation induces apoptosis and turnover of smooth muscle cells, reducing healing and extracellular matrix production (13). The neovascularization that occurs in the wall of the aneurysm, which is caused by the leakage of microvessels, is the cause of the increase in inflammation and the disruption of tissue that occurs concurrently with it (14).
Despite advancements in understanding, substantial information gaps remain. The particular routes that relate systemic inflammatory states to rupture risk are not yet fully identified, despite the fact that experimental models and clinical observations give substantial evidence for the role that inflammation plays in the progression of aneurysms with regard to their progression. Biomarkers like C-reactive protein (CRP) and circulating cytokines may predict aneurysm instability, but their sensitivity and specificity are insufficient for therapeutic use (15). Similarly, PET utilizing fluorodeoxyglucose (FDG) to detect wall inflammation is promising but not yet established in clinical practice (16). A further factor that makes the interpretation of inflammatory contributions more difficult is the variability that exists across the different types of aneurysms. For example, intracranial aneurysms and AAA exhibit different biological characteristics (17). A recent study reveals that molecular and inflammatory markers can be used to forecast the risk of rupture; however, the current guidelines for the management of aneurysms center on the measurements of the aneurysm (18). Diagnostic and therapy efforts must be guided by a full appraisal of current knowledge. This gap between mechanistic understanding and clinical application underscores the necessity for this review.
This review synthesizes existing evidence on chronic inflammation and aneurysm wall stability and rupture risk. Immune cell invasion, cytokine signaling, extracellular matrix disintegration, and smooth muscle cell dysfunction will be highlighted in aneurysm biology. We will also examine systemic inflammatory comorbidities in relation to the development and progression of aneurysms. The review will conclude with therapeutic implications, including the potential for anti-inflammatory treatments to alter disease trajectory and enhance patient outcomes. And it addresses these areas to bridge pathophysiological understanding with clinical practice, improving risk assessment and aneurysmal disease management.
Methodology
A comprehensive literature search in the PubMed, Web of Science, Science Direct, and Cochrane databases utilizing the medical topic headings (MeSH) and relevant keywords such as ‘chronic’, ‘inflammation’, ‘aneurysm’, ‘aneurysm wall stability’, ‘rupture risk’, and a combination of all available related terms was performed on January 22, 2024. All relevant peer-reviewed articles involving human subjects and those available in the English language were included. Using the reference lists of the previously mentioned studies as a starting point, a manual search for publications was conducted through Google Scholar to avoid missing any potential studies. There were no limitations on date, publication type, or participant age.
Discussion
General Interpretation and Summary of Findings
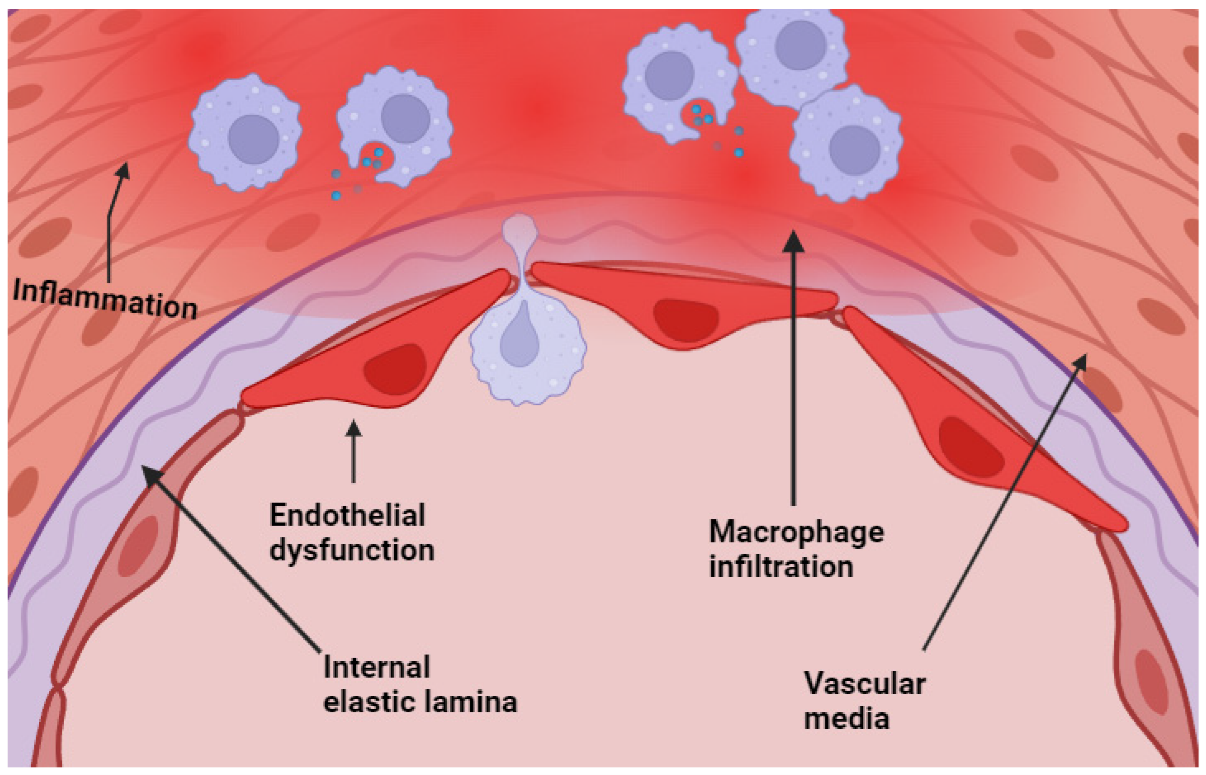
Chronic inflammation plays a critical role in the pathogenesis of aneurysms and molding wall stability and rupture risk (19). It has been proven in a significant number of publications that inflammatory processes have a major influence on the biomechanical and biological integrity of the arterial wall. This occurrence may be due to the presence of macrophages, lymphocytes, and neutrophils in the cerebral and AAA (Figure 1) (20). Cerebral and AAA, as well as the elevated quantities of inflammatory cytokines and proteolytic enzymes. These findings show that aneurysm rupture is the result of inflammation-driven structural degradation as well as mechanical dilation (21). MMPs and other proteases destabilize the extracellular matrix and cause elastin fragmentation, collagen depletion, and vascular smooth muscle cell death during inflammation (22). Hypoxia and unstable microvasculature from aneurysm wall neoangiogenesis cause bleeding and structural weakening. This cycle of inflammation and deterioration enhances wall instability (22). According to numerous studies, systemic inflammation can be recognized, and biomarkers such as CRP and IL-6 have been linked to the establishment of aneurysms and the risk of rupture (23). A comprehensive rupture risk model that incorporates inflammatory conditions, biomechanical stressors, and systemic risk factors is more accurate (24). This study has significant repercussions for the practices of surveillance, patient classification, and the development of medicines that modulate inflammation. Systemic health and illnesses related to chronic inflammation should be studied along with local vascular system changes. According to epidemiological studies, chronic inflammatory illnesses such as rheumatoid arthritis, systemic lupus erythematosus, and chronic obstructive pulmonary disease are linked to brain and abdominal aneurysms (25). The existence of a systemic inflammatory burden may render the vascular environment more susceptible to the development of aneurysms. This observation is true regardless of whether or not hypertension or tobacco use are contributing factors (26). The concept of low-grade chronic inflammation, which is linked to metabolic syndrome and obesity, is additionally gaining more attention as research continues to be conducted. Adipose tissue-derived cytokines create a pro-inflammatory milieu that lowers vascular system resilience (27). Our findings demonstrate the need to include systemic inflammatory states in aneurysm development risk models because they can exacerbate local inflammatory activity in the aneurysm wall. The findings of recent studies have indicated that the metabolites produced by the gut microbiota can have an effect on the systemic inflammatory responses, which in turn may have an effect on the biology of aneurysms and the remodeling of blood vessels (28). Lifestyle change, targeted medication, and microbiome-directed methods may reduce chronic inflammation and reduce aneurysm rupture risk in addition to surgical and endovascular procedures; these findings open new therapeutic possibilities (29).
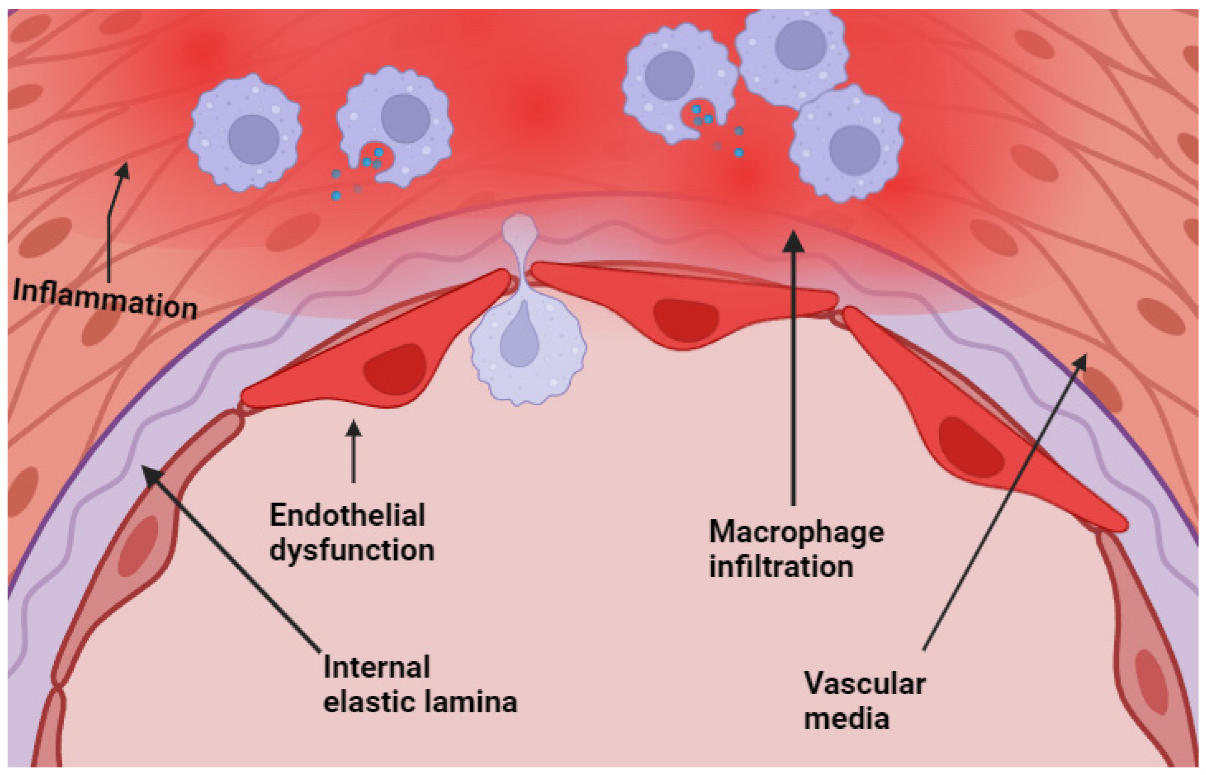
Figure 1: Endothelial dysfunction with macrophage infiltration at the level of the media of the cerebral arteries (20).
Comparison of First Key Significant Factor: Inflammatory Biomarkers
The analyzed studies found a strong link between systemic inflammatory biomarkers and aneurysm rupture risk. AAA and intracranial aneurysm populations with elevated CRP levels have quicker aneurysm expansion and rupture rates (30). A study by Håland et al. in the European Journal of Vascular and Endovascular Surgery examined the association between high-sensitivity C-reactive protein (hs-CRP) and AAA risk in a population-based study. And found that A substantially greater risk of getting AAA was observed in individuals who had elevated levels of hs-CRP (31). These findings highlight the clinical relevance of inflammatory biomarkers as prognostic indicators. Comparable results in Middle Eastern and European populations demonstrate the repeatability of these associations across cohorts. Kami?ska et al. performed the IL-6 Quotient study, evaluating cerebrospinal fluid to serum IL-6 in patients with unruptured intracranial aneurysms (UIA). The IL-6 Quotient was considerably higher in UIA patients compared to controls (1.78 vs. 0.87, p < 0.001), indicating a possible role in aneurysm formation due to local synthesis in the central nervous system (32). Additionally, Serum Interleukin-1 beta (IL-1β) and Interleukin-1 receptor antagonist (IL-1ra) were evaluated in patients with ruptured and unruptured cerebral aneurysms. A substantial link between serum and tissue interleukin-1 levels was identified, with the IL-1 ratio (IL-1.ra/IL-1β) demonstrating high diagnostic accuracy for unstable aneurysms (33). These findings show that inflammatory biomarkers are essential to aneurysm biology regardless of geography or demographics. Study design, biomarker assay variability, and patient heterogeneity may explain variations. Diseases such as infection, inflammatory illness, and metabolic syndrome may also confound systemic biomarkers and aneurysm outcomes. These differences demonstrate the difficulty of interpreting inflammatory indicators as independent predictors. CRP and IL-6 techniques are promising; however, their lack of specificity restricts their therapeutic use. A composite biomarker panel integrating systemic markers with imaging-based wall inflammatory assessments may improve risk classification (34). Emerging multimodal studies reveal that CRP increase and FDG-PET uptake in the aneurysm wall possess greater predictive potential for rupture than either modality independently (35).
Comparison of the Second Key Significant Factor: Vascular Wall Inflammation and Structural Degeneration
Another significant finding in this review article is that local vascular wall inflammation directly destabilizes aneurysms. AAA and intracranial aneurysm histology show macrophage infiltration (Figure 2) (36), smooth muscle cell apoptosis (37), and collagen and elastin fiber degradation (38). On post-mortem evaluation of 120 ruptured AAAs in the United Kingdom, most had significant macrophage infiltration and high matrix metalloproteinase-9 expression, indicating wall rupture (39). In related literature, Wall degeneration, smooth muscle cell loss, lipid accumulation, and calcification are continuously linked to high inflammatory cell infiltration and structural weakness, risking rupture (40). Furthermore, imaging studies provide corroborative evidence. In AAA, the uptake of 18F-FDG, which was evaluated via PET/CT, was found to be associated with cellular and molecular changes that preceded wall disruption and rupture (41). A longitudinal follow-up study by MDI Vergouwen et al. found that gadolinium augmentation of unruptured intracranial aneurysm walls increased the probability of growth or rupture (42). These imaging methods assess local inflammation noninvasively, emphasizing its impact on rupture pathogenesis. A multicenter longitudinal cohort analysis of 455 patients found that gadolinium-enhanced aneurysm wall imaging increased the chance of growth or rupture, although this was not independent of aneurysm size, limiting its predictive power (43). In addition to histology and imaging, growing research emphasizes the need for understanding vascular wall inflammation as a dynamic and heterogeneous process instead of a body-wide pathogenic disease. The geographic heterogeneity that exists inside the aneurysm wall may cause certain sections to demonstrate severe inflammatory infiltration and significant degradation of the extracellular matrix, while other regions may remain unaffected. This is because of the fact that the aneurysm wall is composed of a number of different regions (44). The uneven distribution complicates risk evaluation, and the importance of fault-finding procedures that are focused, high-resolution, and focal is stressed as a result of this. According to long-term investigations, aneurysm wall inflammation changes throughout time. Systemic inflammation, hemodynamic stressors, and local microenvironmental factors, including hypoxia, provoke this oscillation (45). One possible explanation for why some aneurysms with wall inflammation remain stable for years while others rupture immediately with similar imaging data is that these temporal alterations are responsible for the difference (40). Additionally, the interaction between arterial wall inflammation and ILT is of growing attention due to its importance. ILT is physiologically active and releases proteolytic enzymes and inflammatory mediators into the aneurysm wall. This causes localized hypoxia, which leads to the death of smooth muscle cells and the dissolution of the extracellular matrix. As a result, the organization and permeability of the ILT may have an effect on the inflammatory burden of the wall, which makes the assessment of the risk of rupture more difficult (40). Advanced molecular imaging tracers that target integrins, macrophage-specific receptors, or MMPs may improve aneurysm inflammation specificity (46). Similar computational methods are being developed to predict rupture more accurately (47). These models incorporate imaging-derived wall stress, characteristics of the inflammatory response, and inflammatory markers. For the purpose of capturing the multifaceted character of vascular wall instability, these advancements highlight the necessity of a multidisciplinary approach that brings together pathology, imaging science, and computer modeling (48). This evidence suggests that local vascular wall inflammation is crucial to aneurysm instability, although it is ideally assessed with biomechanical stress models and systemic biomarker profiles (49). The variety of study results shows that no single metric or imaging modality may fully capture rupture risk.
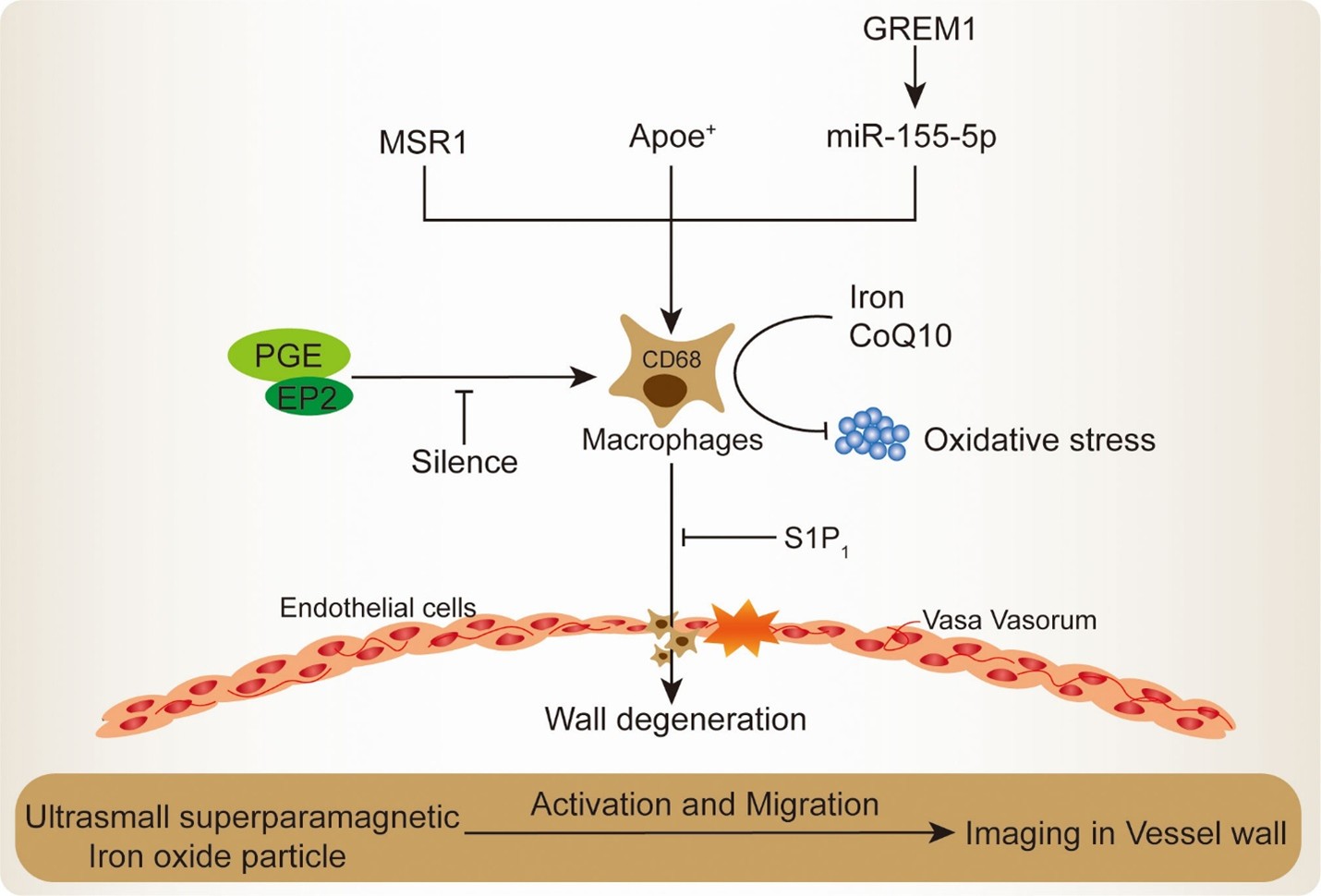
Figure 2: Mechanisms of macrophage recruitment and activation in IA lesion (36).
Limitations of Current Evidence and Recommendations for Future Research and Practice
There are limitations to the evidence that inflammation causes aneurysm rupture. The temporal relationships between inflammation and rupture risk need to be confirmed through prospective longitudinal studies that make use of standardized imaging and biomarker approaches. It is often difficult to conduct direct comparisons because of the variety that occurs across the many studies. Different imaging methods, diagnostic thresholds, biomarker assays, and inclusion criteria that were used in the studies may significantly impact the results. Numerous studies focus independently on AAA or intracranial aneurysms, whereas few compare their inflammatory processes. Comparative studies in this context could elucidate whether the identified variations arise from methodological variance or differing anatomical locations. Generalizability is another limitation; however, some investigations have been conducted on individuals from South Asia, Africa, and the Middle East, although these investigations have been limited. The majority of these studies have focused on Western populations. Genetics, environment, and lifestyle choices affect inflammation; thus, future research must be expanded for wider application. Diabetes, autoimmune disease, and persistent infection can complicate connections, requiring cautious study design (50).
Policymakers should consider adding inflammatory biomarkers to surveillance strategies for high-risk populations in national aneurysm screening programs (51). Clinically, risk stratification models should include multimodal assessment of wall inflammation using systemic biomarkers and imaging. Aneurysm growth and rupture risk reduction by anti-inflammatory treatments, including statins, doxycycline, and new biologics (52), have to be assessed in well-designed prospective cohort studies and randomized controlled trials. Molecular and genetic processes of inflammation may be the subject of translational research, which may lead to the discovery of new therapy targets. A multidisciplinary strategy is needed to connect mechanistic knowledge to treatment. Using biomarker science, sophisticated imaging, and molecular biology, aneurysm care can move from structural to biological drivers of rupture.
Conclusion
Growing evidence suggests that inflammatory mechanisms impair structural integrity equally; however, aneurysm size and growth rate still dictate therapeutic decision-making. Size-based techniques are limited; thus, these findings support biology-driven risk assessment. Using systemic inflammatory biomarkers, such as CRP and IL-6, and imaging evidence of local vascular inflammation appears to be a feasible approach for non-invasive risk classification. Various studies underscore the need for standardized methods and prospective validation across diverse groups. Clinically, these findings indicate an improvement in practice. Targeted therapy evaluation and molecular pathway understanding require high-quality longitudinal and translational research. Aneurysm wall instability and rupture are caused by chronic inflammation. Risk prediction and patient outcomes are best achieved with an integrated strategy that combines structural, biomechanical, and biological aspects.
Disclosure
Conflict of interest
There is no conflict of interest.
Funding
No funding.
Ethical consideration
Non applicable.
Data availability
Data that support the findings of this study are embedded within the manuscript.
Author contribution
All authors contributed to conceptualizing, data drafting, collection and final writing of the manuscript.